Atoms & Molecules echapter — The Biology Primer
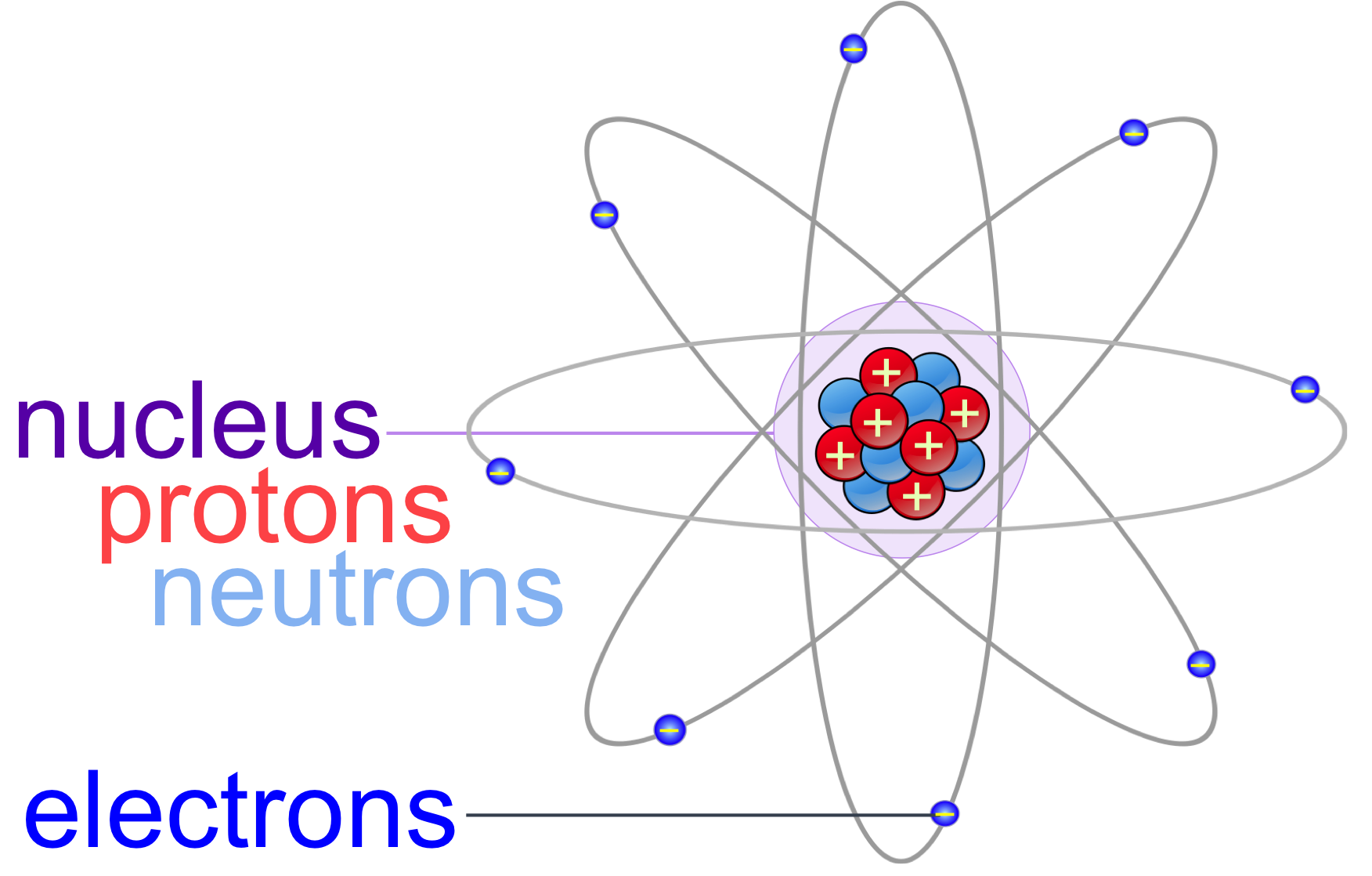
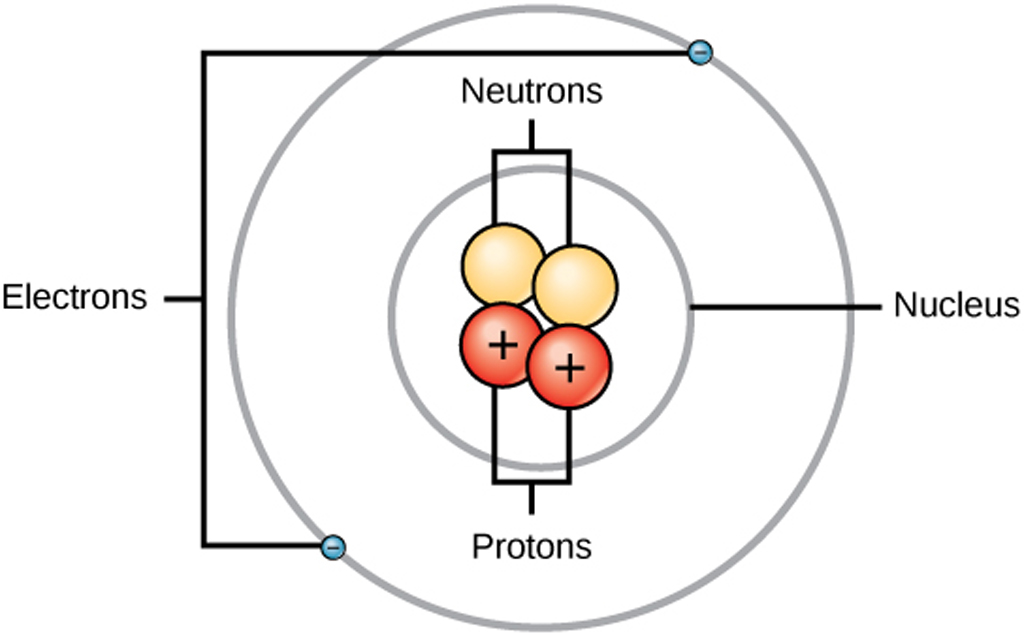
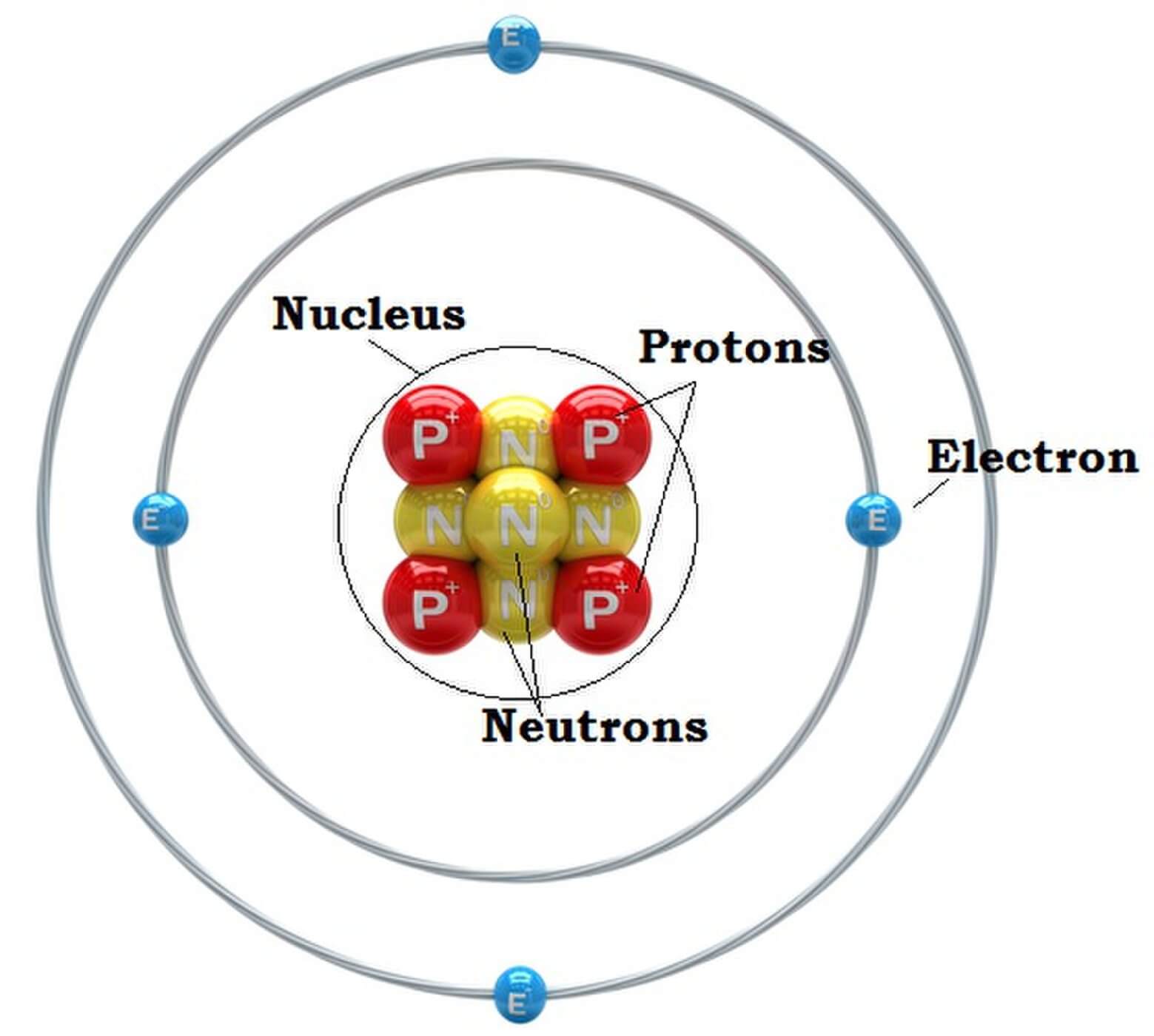
You can see that each part of the atom is labeled with a "+", "-", or a "0." Those symbols refer to the charge of the particle. Have you ever heard about getting a shock from a socket, static electricity, or lightning? Those are all related to electric charges. Charges are also found in tiny particles of matter.
The Structure of Atoms
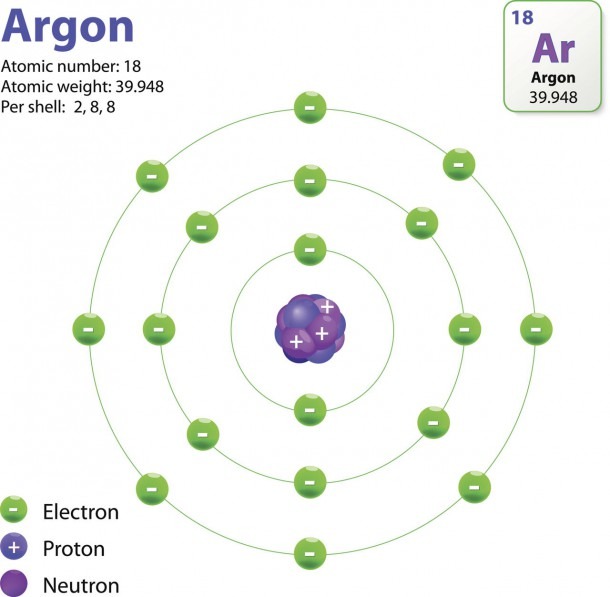
Relative charge. -1. The number of electrons in an atom is always the same as the number of protons, so atoms are electrically. neutral. overall. Atoms can lose or gain electrons. When they do.
The Structure Of An Atom Explained With A Labeled Diagram Best Diagram Collection
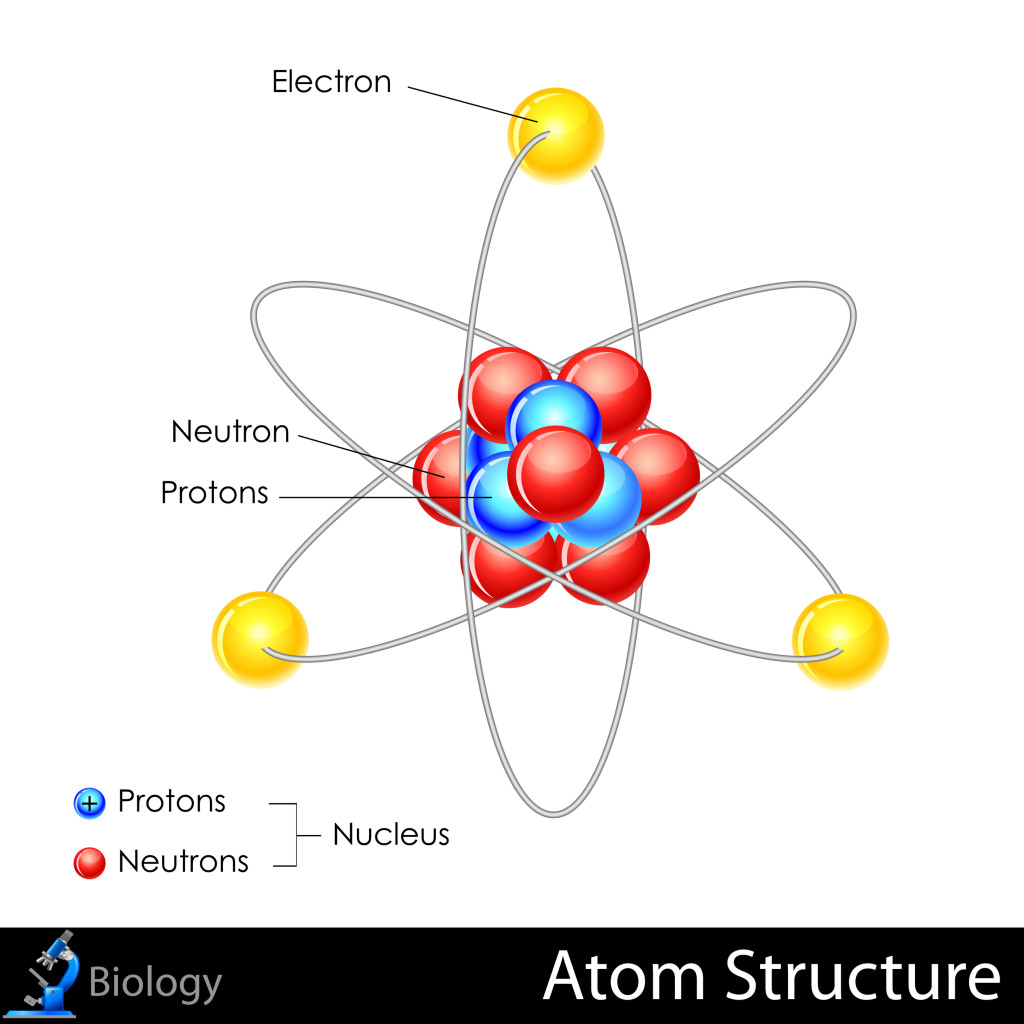
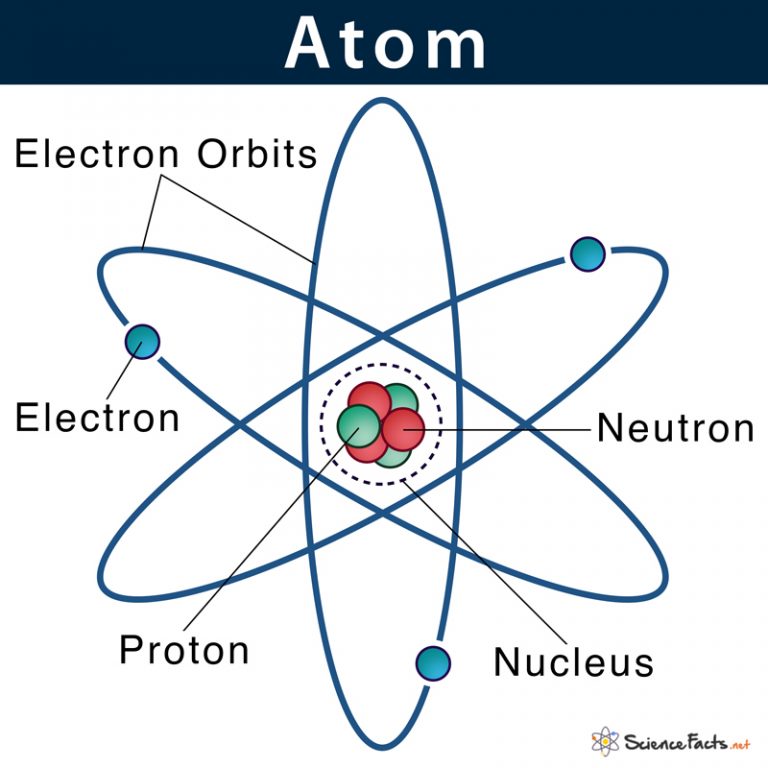
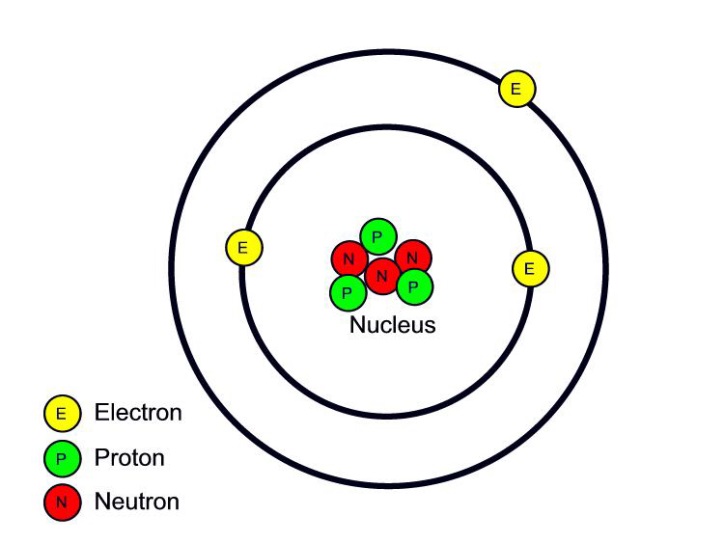
Key Takeaways: Model of the Atom. An atom is a building block of matter that cannot be broken apart using any chemical means. Nuclear reactions can alter atoms. The three parts of the atom are protons (positively charged), neutrons (neutral charge), and electrons (negatively charged). Protons and neutrons form the atomic nucleus.
Atom Definition, Structure & Parts with Labeled Diagram
Most of the atom is empty space. The rest consists of three basic types of subatomic particles: protons, neutrons, and electrons.The protons and neutrons form the atom's central nucleus. (The ordinary hydrogen atom is an exception; it contains one proton but no neutrons.) As their names suggest, protons have a positive electrical charge, while neutrons are electrically neutral—they carry.
/GettyImages-141483984-56a133b65f9b58b7d0bcfdb1.jpg)
35 Label The Parts Of The Atom In The Diagram Below Labels For Your Ideas
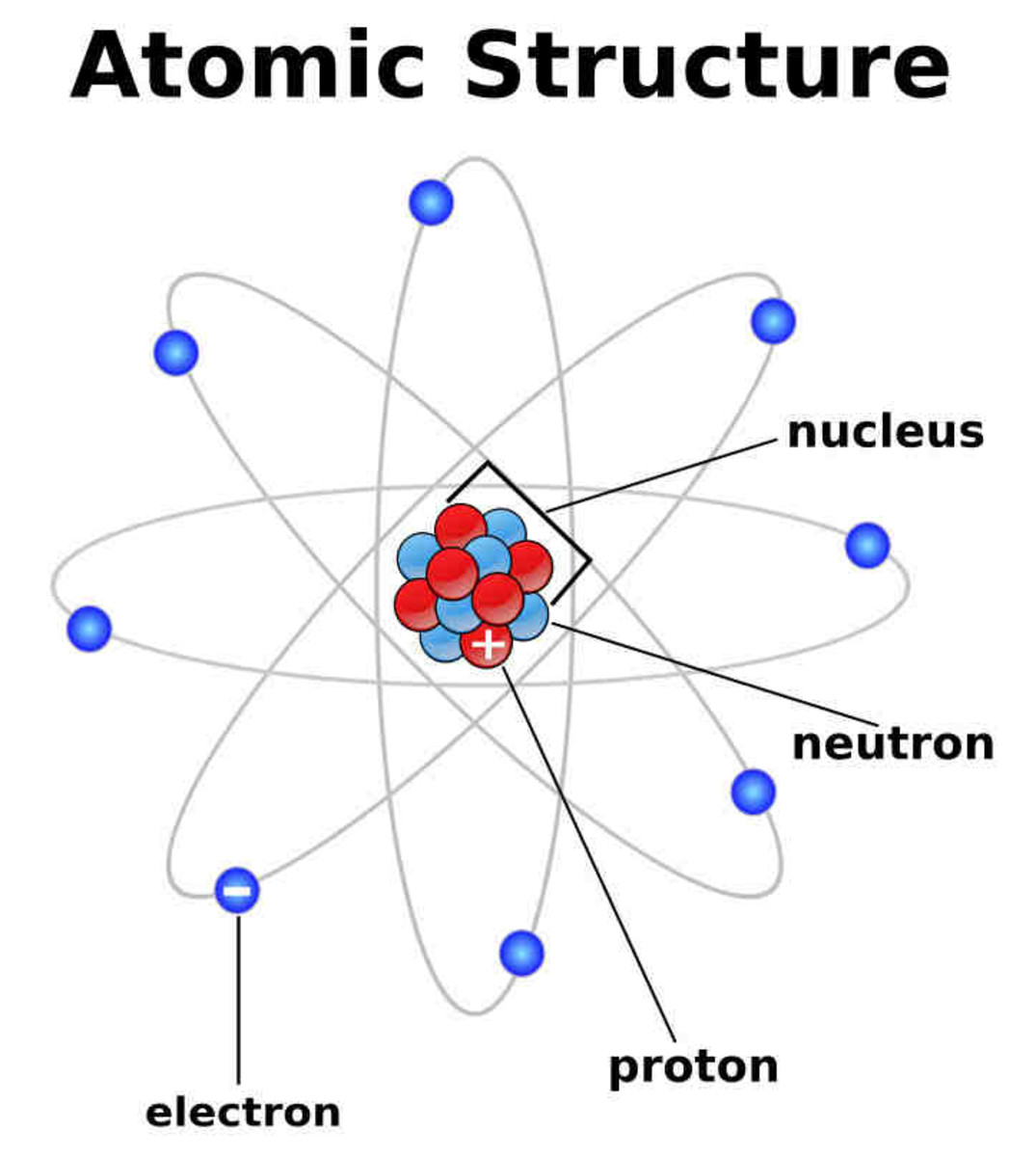
This entry was posted on January 20, 2017 by Anne Helmenstine (updated on November 19, 2023) The three main parts of an atom are protons, neutrons, and electrons. The atom is the basic building block of matter. Atoms combine to form pure elements, compounds, and complex forms like computers and phones. Atoms are the smallest particle of matter.
Structure of an Atom Structure & Use of Electron & Proton in Electronics
Chemical Symbols. A chemical symbol is an abbreviation that we use to indicate an element or an atom of an element. For example, the symbol for mercury is Hg (Figure \(\PageIndex{3}\)). We use the same symbol to indicate one atom of mercury (microscopic domain) or to label a container of many atoms of the element mercury (macroscopic domain).
Atoms and Atomic Structure HubPages
Devised by Russian chemist Dmitri Mendeleev (1834-1907) in 1869, the table places elements into columns— groups —and rows— periods —that share certain properties. These properties determine an element's physical state at room temperature—gas, solid, or liquid—as well as its chemical reactivity, the ability to form chemical bonds with other atoms.
atom diagram to label
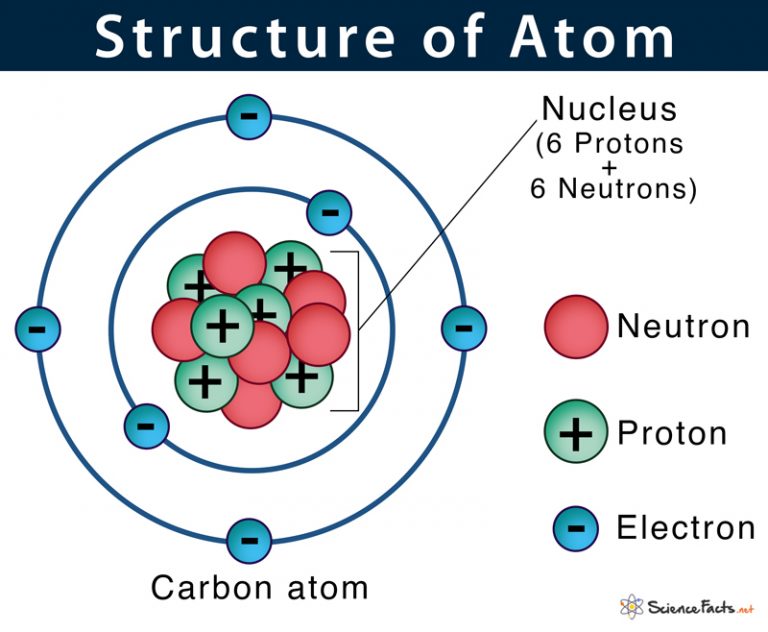
Primarily, the atomic structure of matter is made up of protons, electrons and neutrons. The protons and neutrons make up the nucleus of the atom, which is surrounded by the electrons belonging to the atom. The atomic number of an element describes the total number of protons in its nucleus. Neutral atoms have equal numbers of protons and.
Atomic structure WGHS Junior Science
Written By Pavithra VG Last Modified 22-06-2023 The Structure of an Atom: Parts, Diagram, Examples Anything that has mass and occupies space is called matter. The matter is made up of atoms. Atomic structure is the structure of an atom that consists of a nucleus (the centre), protons (positively charged), and neutrons (neutral).
What is an Atom? Definitions & Examples Let us learn Basics News Bugz
Because the sum of the numbers of protons and neutrons equals the mass number, 127, the number of neutrons is 74 (127 − 53 = 74). Since the iodine is added as a 1− anion, the number of electrons is 54 [53 - (1-) = 54]. Exercise 2.2.1 2.2. 1. An ion of platinum has a mass number of 195 and contains 74 electrons.
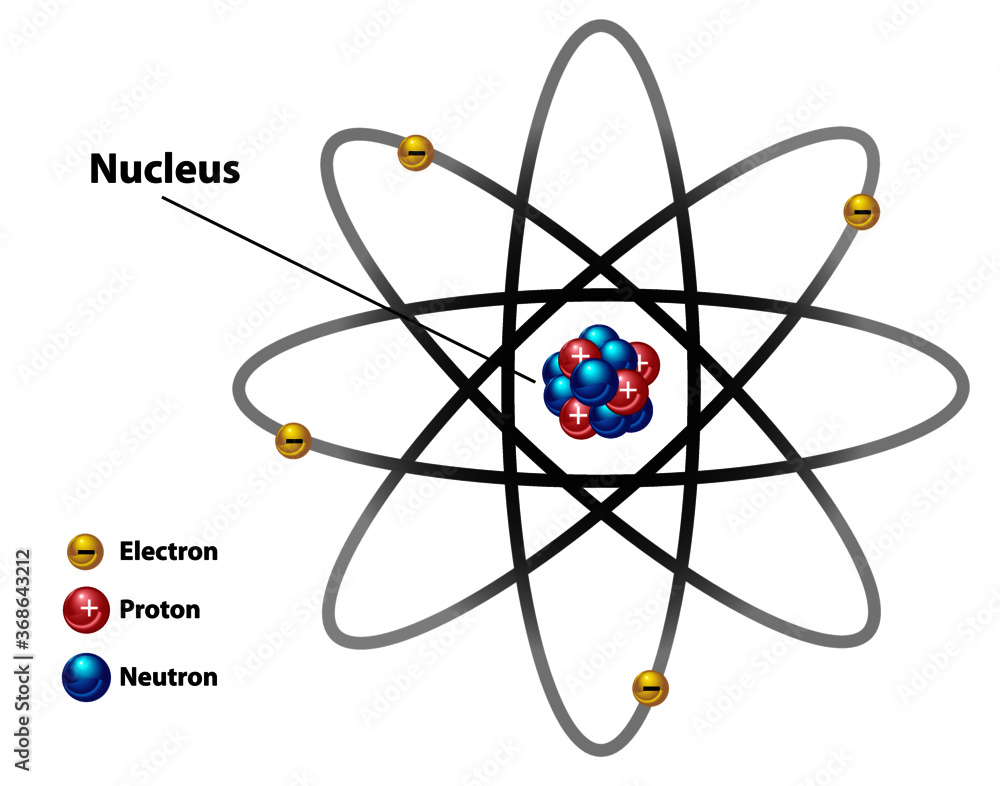
Atomic nucleus diagram labeled with electron, proton, and neutron. Stock Vector Adobe Stock
Recall that a molecular formula shows the number of atoms of each element that a molecule contains. A molecule of water contains two hydrogen atoms and one oxygen atom, so its formula is \(\ce{H_2O}\). A molecule of octane, which is a component of gasoline, contains 8 atoms of carbon and 18 atoms of hydrogen.

Atomic Structure Broad Learnings
I go over how to draw and label and atom.Website: https://sites.google.com/view/andrewhaskell/home

Energy Mind Map
And while ancient magi and philosophers conceived of a world composed of four or five elements - earth, air, water, fire (and metal, or consciousness) - by classical antiquity, philosophers began.
Subatomic Makeup Of An Atom Makeupview.co
An ion of an atom is one in which the number of protons and electrons is not the same. If there are more protons than electrons, an atomic ion has a positive charge and is called a cation. If there are more electrons than protons, the ion has a negative charge and is called an anion.

Structure of an atom universalxoler
Mass: The majority of an atoms' mass comes from the protons and neutrons that make up its nucleus. Electrons are the least massive of an atom's constituent particles, with a mass of 9.11 x 10-31.
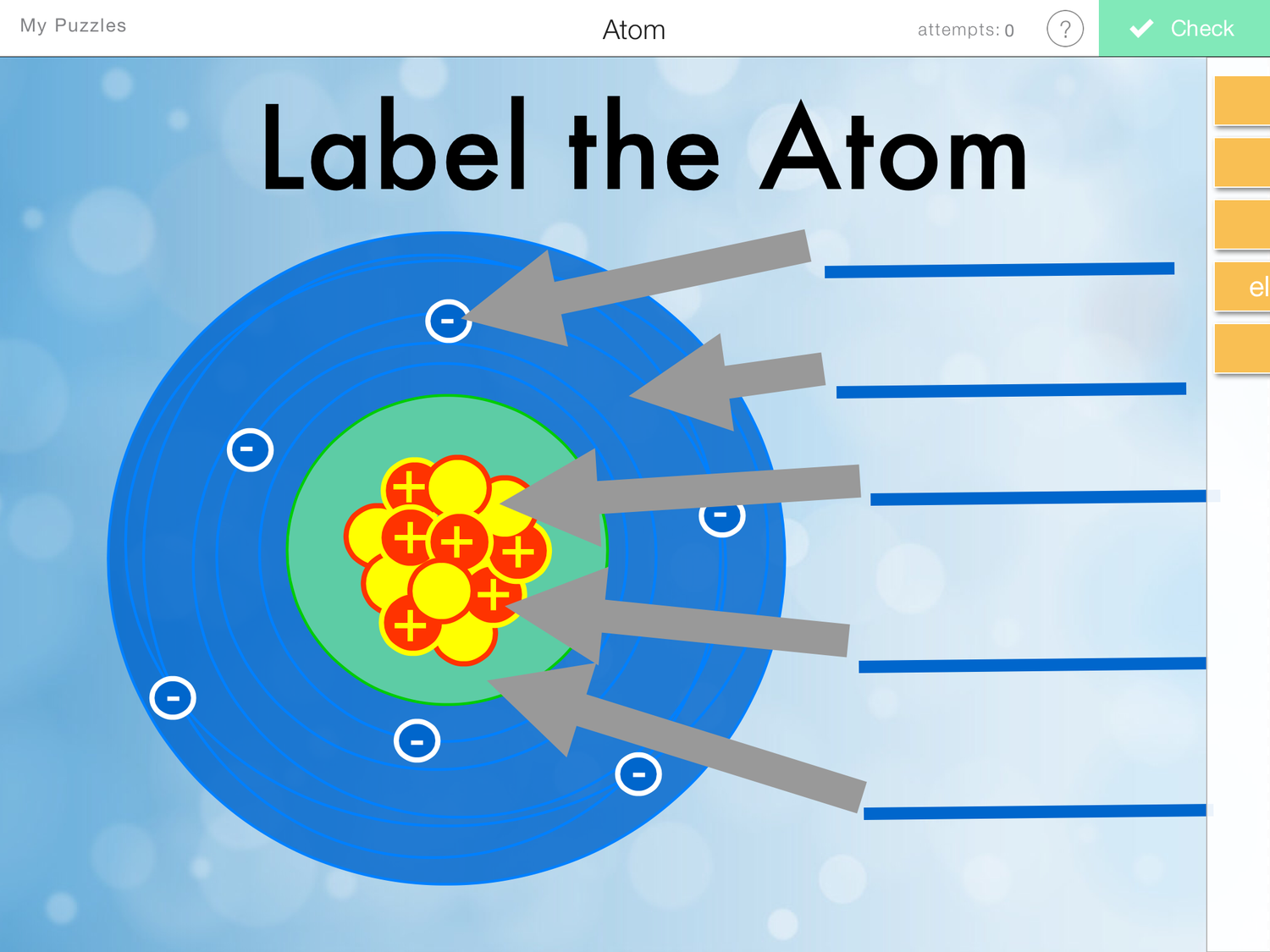
Label Parts of an Atom — Learning in Hand with Tony Vincent
An atom that gains one or more electrons will exhibit a negative charge and is called an anion. Positively charged atoms called cations are formed when an atom loses one or more electrons. For example, a neutral sodium atom (Z = 11) has 11 electrons. If this atom loses one electron, it will become a cation with a 1+ charge (11 − 10 = 1+).