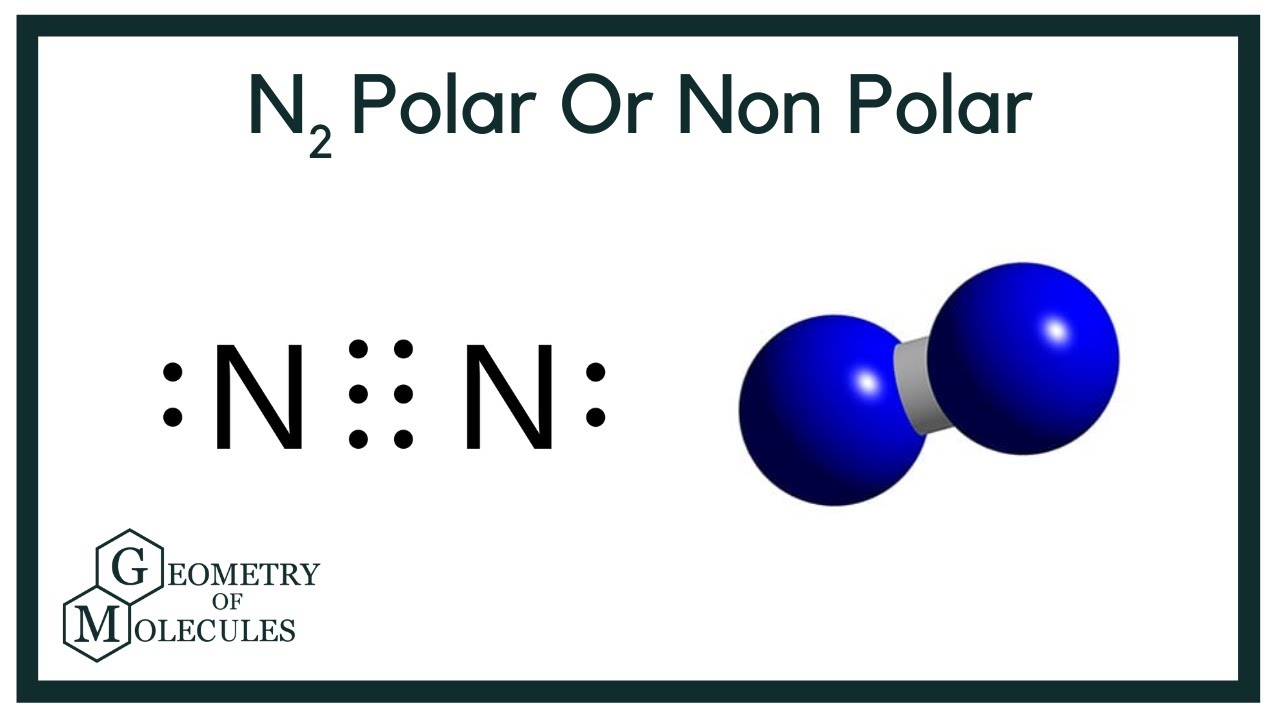
Is N2 Polar Or Nonpolar?
To determine if CH 2 Cl 2 (dichloromethane) is polar or nonpolar, we need to first determine its geometry. This presumes knowing the rules for drawing a correct Lewis structure and you can find more details about Lewis structures here. Carbon is the central atom: There are 4 + 2 + 2×7 = 20 electrons, and 8 have been used to make four bonds.

Best Explanation Ch2cl2 Polar Or Nonpolar N01 Science Education And
The molecule is symmetric. The two oxygen atoms pull on the electrons by exactly the same amount. Propane is nonpolar, because it is symmetric, with H atoms bonded to every side around the central atoms and no unshared pairs of electrons. Exercise 4.12. 1. Label each of the following as polar or nonpolar.

Determine the Electron Geometry of Ch2cl2
Is ch2cl2 polar or is it non-nonpolar and whether it is ionic or covalent all these facts and characteristics have been discussed in detail in this article. We know ch2cl2 is a tetrahedral molecule and not all tetrahedral molecules are polar. But ch2cl2 is a polar molecule and almost all the tetrahedral molecules have a bond angle of 109.5 degrees.

Is CH2Cl2 Polar or Nonpolar? (Dichloromethane) YouTube
Is CH2Cl2 Polar or Non-Polar? (Dichloromethane) Dichloromethane is a polar solvent. This means that it has a dipole moment, which is a measure of how strongly its molecules are attracted to one another.

Polar and Nonpolar Covalent Bonds Characteristics & Differences
CH3Cl Polar or Nonpolar. To determine if CH 3 Cl is polar or nonpolar, we need to first determine its geometry. This presumes knowing the rules for drawing a correct Lewis structure and you can find more details about Lewis structures here. CH 3 Cl has 4 + 3 + 7 = 14 valence electrons. Carbon goes in the middle and is bonded to three hydrogen.

Polar and Nonpolar Molecules
CH2Cl2 is a polar molecule due to its tetrahedral geometrical shape and difference between the electronegativity of Carbon, Hydrogen and Chlorine atoms. This develops a dipole moment across C-Cl and C-H bonds and the entire molecule results in a net 1.67 D dipole moment. Methyl Chloride is majorly produced by the emission through industries.
Ch2cl2 3d Structure
The molecule is symmetric. The two oxygen atoms pull on the electrons by exactly the same amount. Propane is nonpolar, because it is symmetric, with H atoms bonded to every side around the central atoms and no unshared pairs of electrons. Exercise 4.12. 1. Label each of the following as polar or nonpolar.

Solved Question 9 (1 Point) What Are The Polarities Of CH...
It is polar because of the presence of two chloro groups but is not miscible with water; however, it does show miscibility with various organic solvents such as chloroform, carbon tetrachloride, hexane, benzene, ethyl acetate, and alcohols.

Is ch2cl2 polar Why, How, When And Detailed Facts
Is CH2Cl2 Polar or Nonpolar? (Dichloromethane) Wayne Breslyn 726K subscribers Join Subscribe Subscribed 373 Share 50K views 3 years ago Learn to determine if CH2Cl2 (Dichloromethane) is polar.
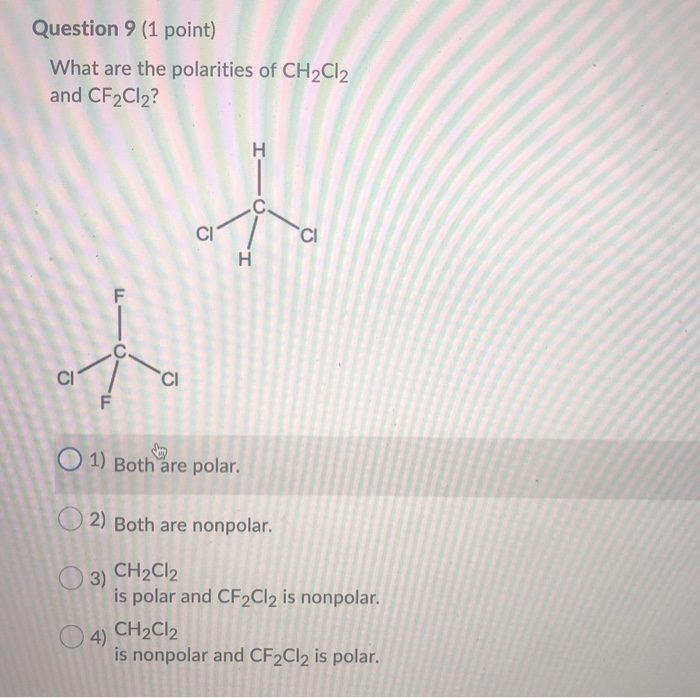
Solved Question 9 (1 Point) What Are The Polarities Of CH...
Molecular Polarity. To determine if a molecule is polar or nonpolar, it is frequently useful to look at Lewis structures. Nonpolar compounds will be symmetric, meaning all of the sides around the central atom are identical - bonded to the same element with no unshared pairs of electrons.Polar molecules are asymmetric, either containing lone pairs of electrons on a central atom or having atoms.
Ch2cl2 Molecular Shape My XXX Hot Girl
Is CH2Cl2 Polar or Nonpolar? All You Need to Know Dichloromethane - Is It Polar or Nonpolar? July 17, 2021 Whether CH2Cl2 is polar or nonpolar is still a question of many. Do we see any separation of any electronic charge in this compound, which led the molecule to have both positive and negative ends?

MakeTheBrainHappy Is CH2Cl2 Polar or Nonpolar?
Hey Guys!In this video, we are going to determine the polarity of Dichloromethane having a chemical formula of CH2Cl2. It is made of one Carbon atom, two Chl.

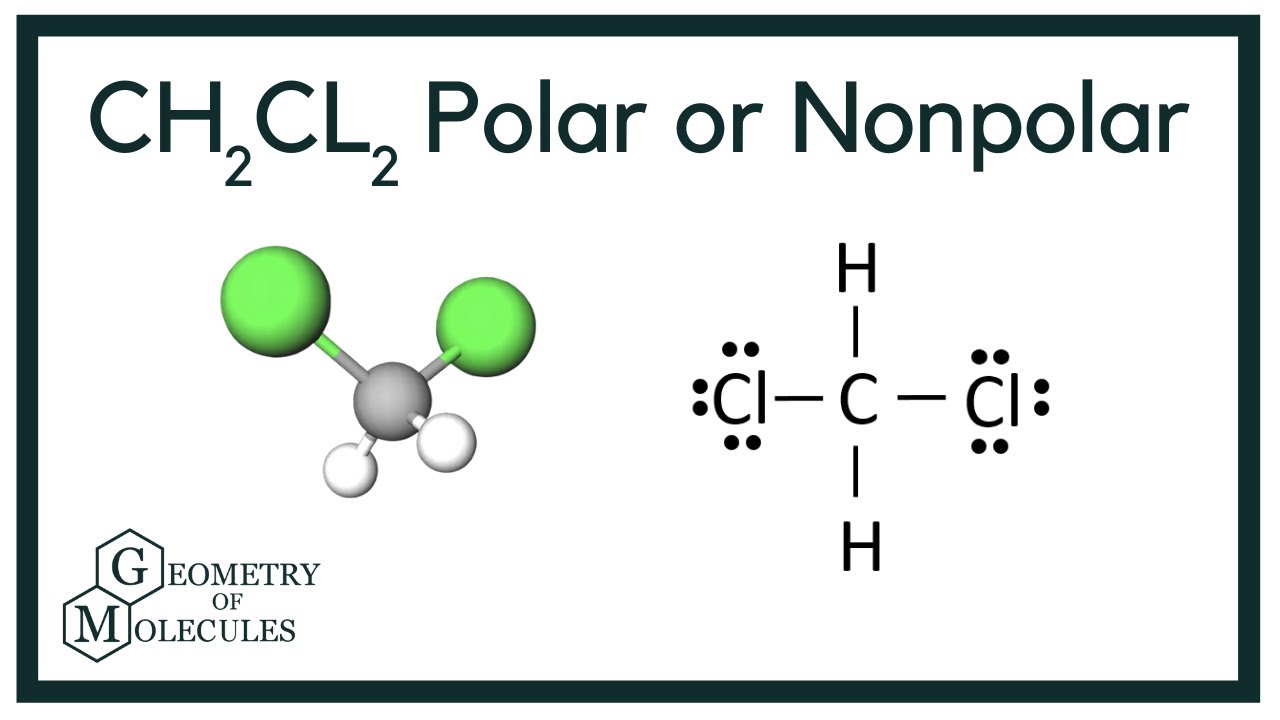
CH2Cl2 Lewis Structure Lewis Dot Structure for CH2Cl2 Dichloro
I assumed, since the reason for CHX2ClX2 C H X 2 C l X 2 being polar is apparently due to the 109.5° angles from the tetrahedral shape, that this means that the surrounding atoms in all tetrahedral-shaped compounds are not placed directly opposite of each other and that is why the dipoles can't cancel each other out. Am I understanding this wrong?
13+ Lewis Structure For Ch2Cl2 Robhosking Diagram
Dichloromethane ( DCM, methylene chloride, or methylene bichloride) is an organochlorine compound with the formula C H 2 Cl 2. This colorless, volatile liquid with a chloroform -like, sweet odor is widely used as a solvent. Although it is not miscible with water, it is slightly polar, and miscible with many organic solvents. [12] Occurrence
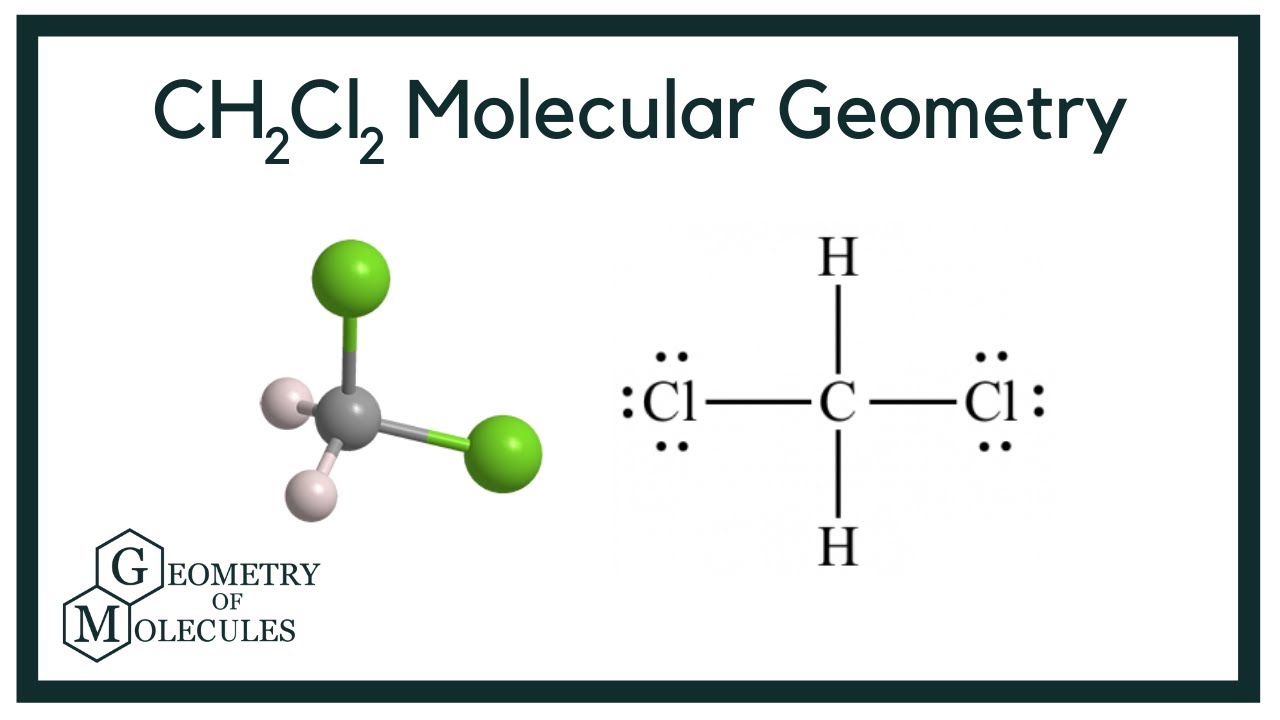
CH2Cl2 Molecular Geometry, Bond Angles & Electron Geometry
CH2Cl2 is a POLAR molecule because the C-Cl bonds present in the molecule are polar, which causes the partial positive (ẟ+) and partial negative (ẟ-) charge to appear on the molecule. These ẟ+ and ẟ- charges are responsible to make the entire CH2Cl2 molecule polar.

Cloruro de diclorometano deuterado cloroformo deuterado, achtung
Exercise 2.12: Vitamins can be classified as water-soluble or fat-soluble (consider fat to be a very non-polar, hydrophobic 'solvent'. Decide on a classification for each of the vitamins shown below. Exercise 2.13: Both aniline and phenol are insoluble in pure water. Predict the solubility of these two compounds in 10% aqueous hydrochloric acid.