
Scn Lewis Structure
Following steps are required to draw SCN - lewis structure and they are explained in detail in this tutorial. Find total number of electrons of the valance shells of sulfur, nitrogen and carbon atoms and including charge of the -1 charge Total electrons pairs in valence shells Determine center atom from carbon, nitrogen and sulfur atoms

Lewis Dot Structure for elements YouTube
0:00 / 3:24 • Intro SCN- Lewis Structure - How to Draw the Lewis Structure for SCN- (Thiocyanate Ion) Wayne Breslyn 725K subscribers Join Subscribe Subscribed 227K views 10 years ago A.

Lewis Structure SCN plus dipoles, shape, angles, resonance and formal
SCN- Lewis Structure|| Lewis Dot Structure for SCN- ||Thiocyanate ion Lewis Structure#SCN-LewisStructure#LewisStructureforSCN-This video has solved the follo.

Lewis dot structures SCN. Formal charges YouTube
We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 7.9 shows the Lewis symbols for the elements of the third period of the periodic table. Figure 7.9 Lewis symbols illustrating the number of.

RCSB PDB SCN Ligand Summary Page
SCN- Lewis Structure (Thiocyanate Ion) Geometry of Molecules 3.22K subscribers Subscribe Subscribed 4.7K views 1 year ago Lewis Structure Hello guys! Learn the Lewis Structure of SCN- a.
SOLVEDThe thiocyanate ion, \mathrm{SCN}^{}, can form bonds to metals
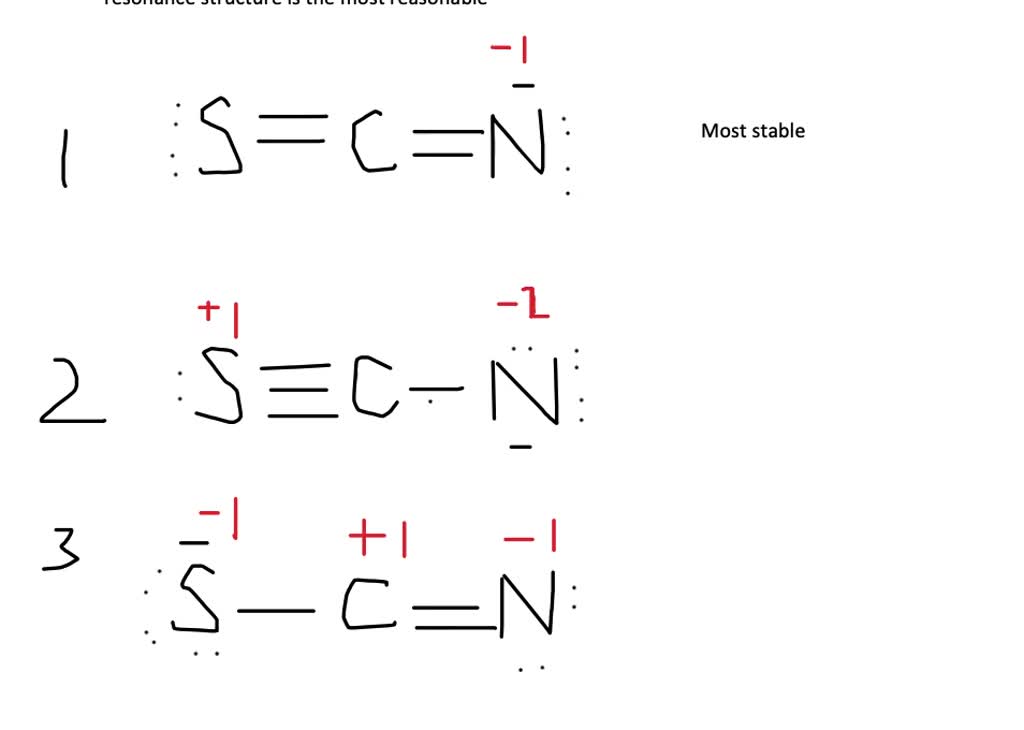
Draw a Lewis dot structure for [SCN]-. Is it one of the ones below? All of these molecules look different, but they fulfill the simple rules of Lewis dot structures. They all fulfill the octet rule and use 16 electrons (15 valence electrons +1 from the -). This demonstrates that in certain cases, molecules can have the same elements but it can.
SCN Lewis Structure, Molecular Geometry, Hybridization and Shape
A Lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. When constructing a Lewis diagram, keep in mind the octet rule, which refers to the tendency of atoms to gain, lose, or share.

SCN Molecular Geometry / Shape and Bond Angles YouTube
A Lewis dot diagram is a visual representation of an atom's valence electrons. In the case of the SCN- ion, the Lewis dot diagram for sulfur would show six dots around the symbol "S," representing its six valence electrons. Similarly, carbon would have four dots, and nitrogen would have five.
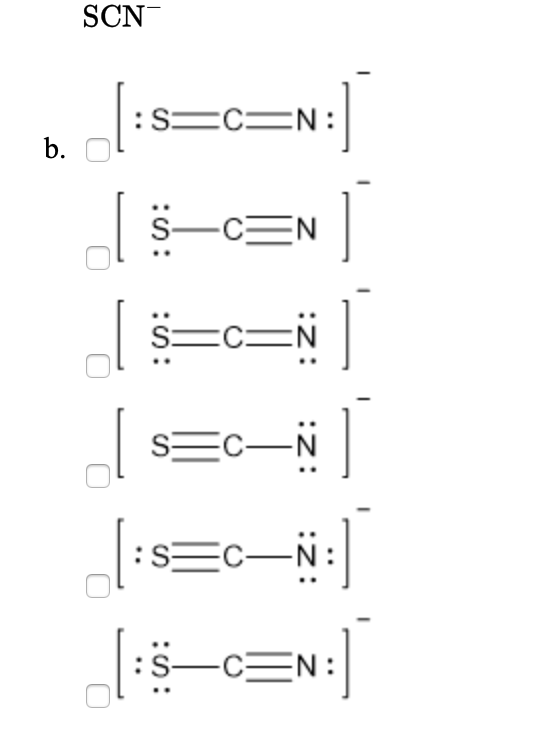
Scn Lewis Structure
Using Lewis Dot Symbols to Describe Covalent Bonding This sharing of electrons allowing atoms to "stick" together is the basis of covalent bonding. There is some intermediate distant, generally a bit longer than 0.1 nm, or if you prefer 100 pm, at which the attractive forces significantly outweigh the repulsive forces and a bond will be formed.
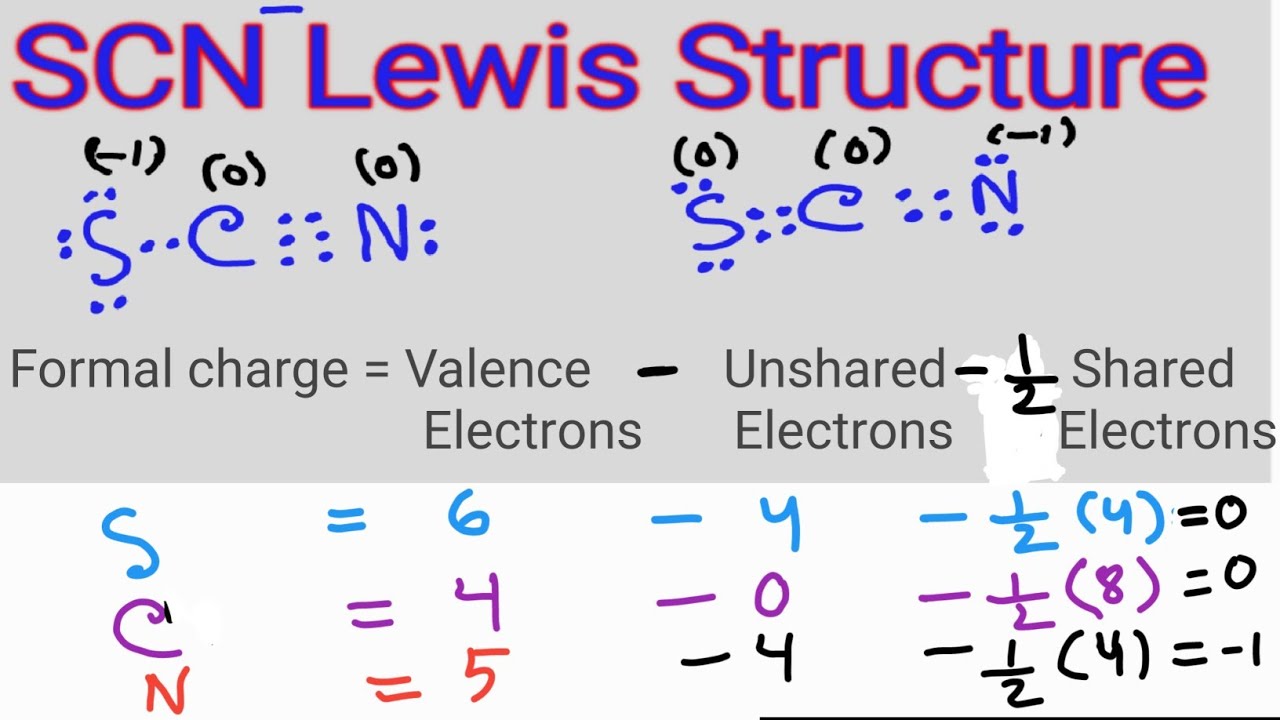
SCN Lewis Structure Lewis Dot Structure for SCN Thiocyanate ion
The Lewis structure for SCN- has 16 valence electrons. SCN- Lewis Structure - How to Draw the Lewis Structure for SCN- (Thiocyanate Ion) Watch on See the Big List of Lewis Structures Transcript: This is Dr. B. We're going to look at the SCN- Lewis structure.

SCN Lewis Structure (Thiocyanate Ion) YouTube
Rome and Latium. Lazio is the region in central Italy that is the center of world history - where Rome is located, and where ancient peoples once flourished. The Latin name Latium indicated the area for many millennia. The region is much larger than its famous capital city, where long before Romulus and Remus were mythically born, the Etruscans.
Lewis Theory of Bonding Chemistry LibreTexts
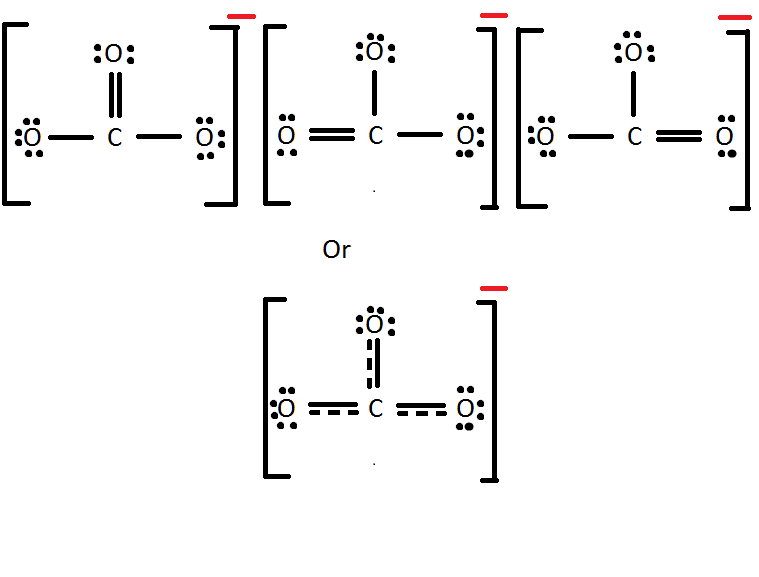
Using Lewis Dot Symbols to Describe Covalent Bonding. The valence electron configurations of the constituent atoms of a covalent compound are important factors in determining its structure, stoichiometry, and properties.. Use the step-by-step procedure to write two plausible Lewis electron structures for SCN.
How to Draw Lewis Dot Structure Grade 11 Chemistry YouTube
This widget gets the Lewis structure of chemical compounds. Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.

(Solved) RbIO2 Draw the Lewis dot structure for RbIO2. Include all
Step 1: Connect the atoms with single bonds. Step 2: Calculate the # of electrons in π bonds (multiple bonds) using formula (1): Where n in this case is 3. Where V = (6 + 4 + 5) - (-1) = 16 , V is the number of valence electrons of the molecule. Therefore, P = 6n + 2 - V = 6 * 3 + 2 - 16 = 4 So, there are : 2 double bonds or a triple bond.

SCN Lewis Structure How to Draw the Lewis Structure for SCN
SCN- lewis structure has a Carbon atom (C) at the center which is surrounded by Sulfur atom (S) and Nitrogen atom (N). There are 2 double bonds between the Carbon (C) & Sulfur (S) atom as well as between Carbon (C) & Nitrogen (N) atom. There is a -1 formal charge on the Nitrogen atom (N).
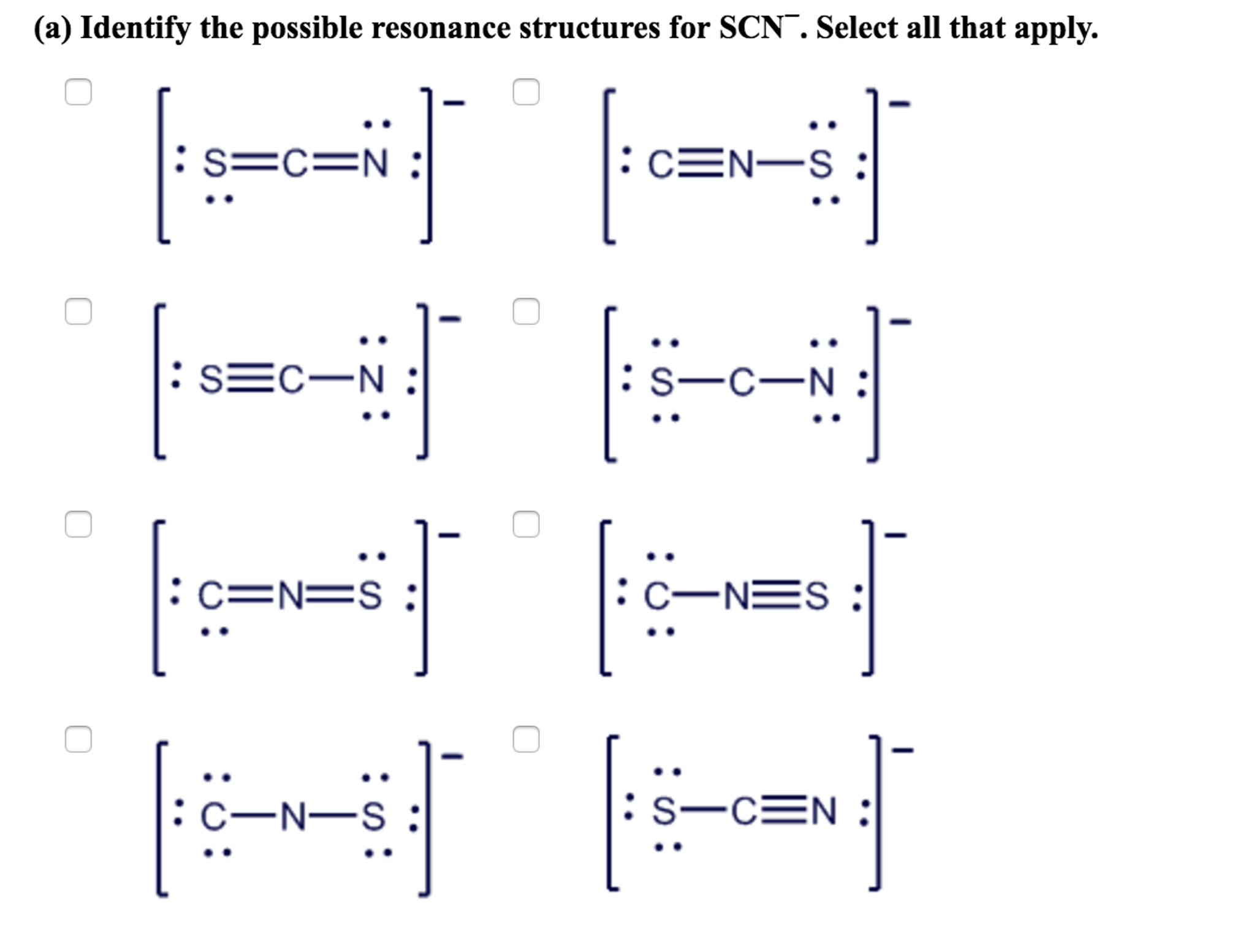
Identify the possible resonance structures for SCN^.
Lewis dot structures: SCN-. Formal charges Diego Troya 5.5K subscribers Subscribe 160 12K views 5 years ago 13-8 This video explains the calculation and use of formal charges by drawing the.