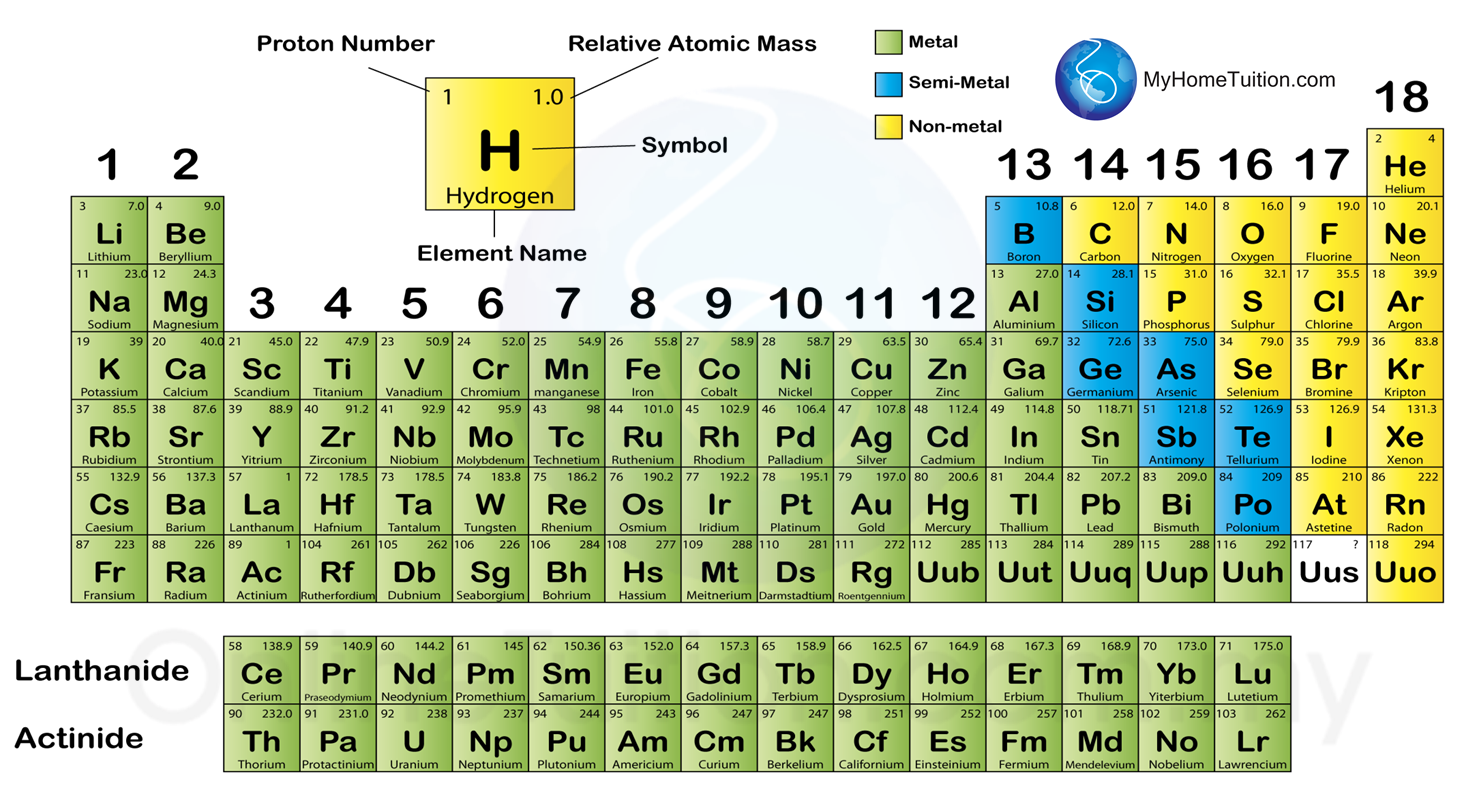
4.4 Classification of Elements in the Period Table SPM Science
What are electron configurations? Electron Configurations are an organized means of documenting the placement of electrons based upon the energy levels and orbitals groupings of the periodic table. The electron configuration for the first 10 elements H 1s1 He 1s2

Cool Element Wallpapers Top Free Cool Element Backgrounds WallpaperAccess
Summary. This update automatically applies Safe OS Dynamic Update to the Windows Recovery Environment (WinRE) on a running PC to address a security vulnerability that could allow attackers to bypass BitLocker encryption by using WinRE. For more information, see CVE-2024-20666.
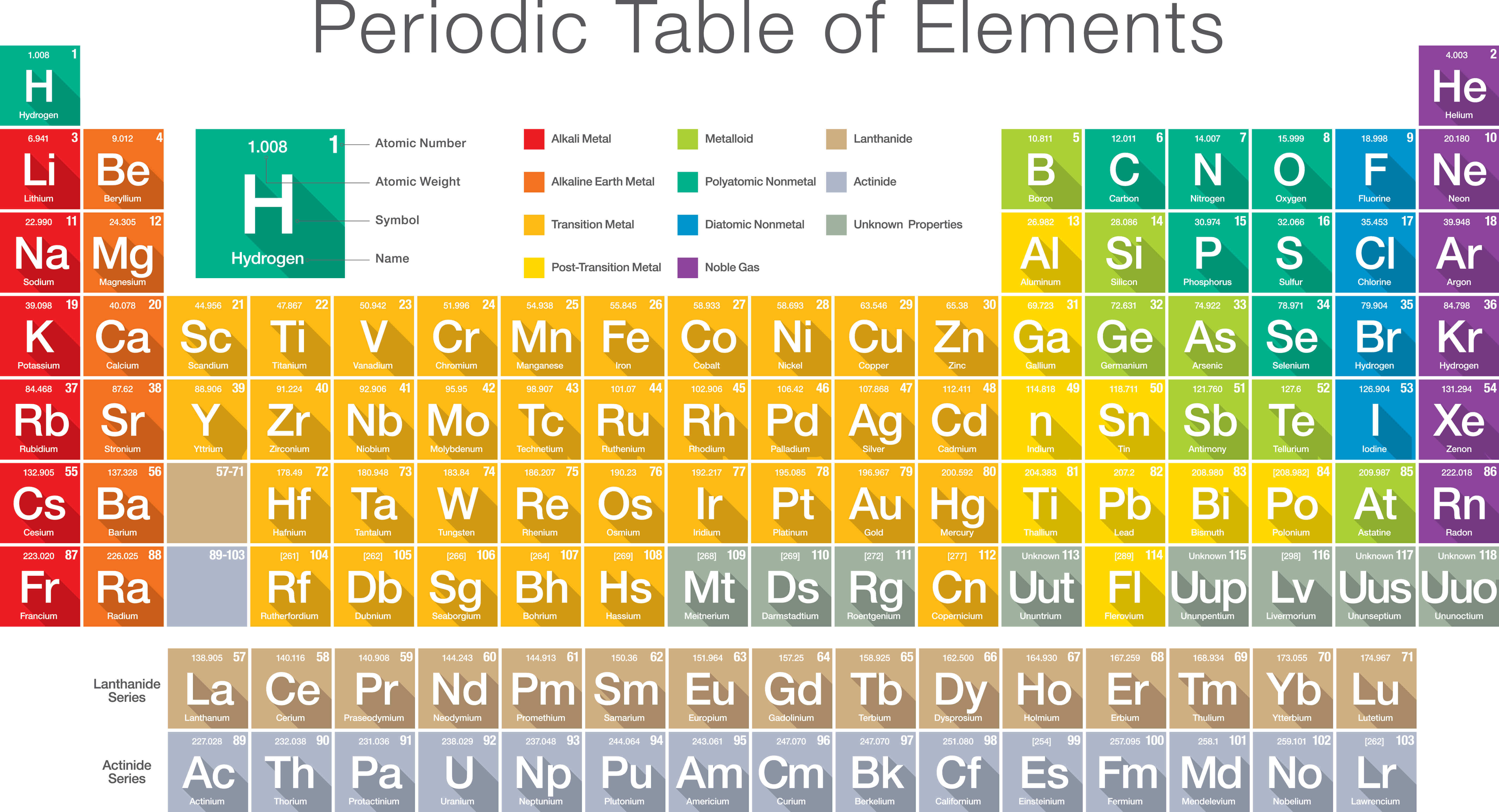
periodic_table_of_the_elements Legends of Learning
In the modern periodic table, the elements are listed in order of increasing atomic number. The atomic number is the number of protons in the nucleus of an atom. The number of protons define the identity of an element (i.e., an element with 6 protons is a carbon atom, no matter how many neutrons may be present).
/periodic-table-of-the-elements-2017--illustration-769723031-5ac10eb6a9d4f9003769784d.jpg)
Element List Atomic Number, Element Name and Symbol
Two-element wattmeters are generally used in delta connected systems. They require two line-line voltages and only two phase currents. Three-element wattmeters are used in wye connected systems. They require three line-neutral voltages and three phase currents.

Best Honda Element Year VehicleHistory
Use the element blocks of the periodic table to find the highest electron orbital. Alternatively, remember group 1 (alkali metals) and group 2 (alkaline earth metals) are s-block, groups 2 through 12 are the d-block, 13 to 18 are the p-block, and the two rows at the bottom of the table (the lanthanides and actinides) are f-block.

Element 26 What is Element 26?
Periodic table of the chemical elements showing the most or more commonly named sets of elements (in periodic tables), and a traditional dividing line between metals and nonmetals. The f-block actually fits between groups 2 and 3; it is usually shown at the foot of the table to save space. Part of a series on the Periodic table Periodic table forms

5 ELEMENT — Брюс Уиллис в глицерине ViVA la Cloud
A modern periodic table arranges the elements in increasing order of their atomic numbers and groups atoms with similar properties in the same vertical column (Figure 2.5.2 2.5. 2 ). Each box represents an element and contains its atomic number, symbol, average atomic mass, and (sometimes) name. The elements are arranged in seven horizontal.
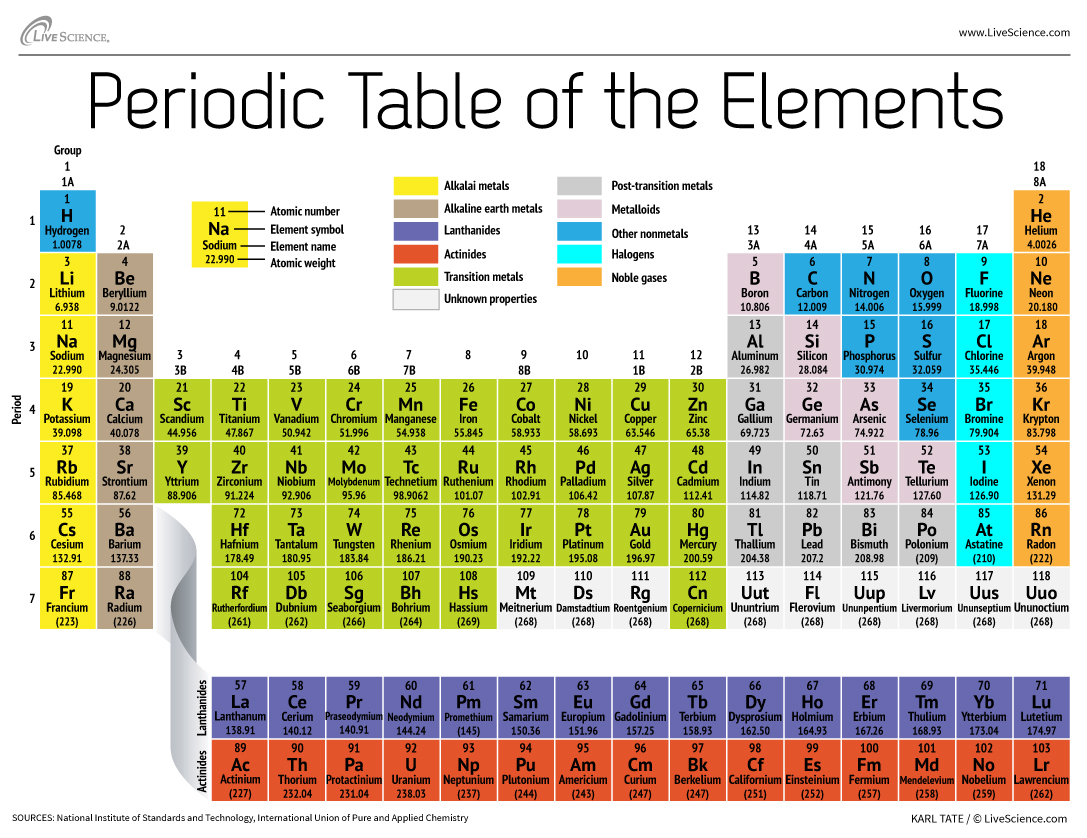
How the Periodic Table groups the elements Live Science
There are 118 elements on the periodic table. Each element is identified by the number of protons in its atoms. This number is the atomic number. The periodic table lists the elements in order of increasing atomic number. Each element has a symbol, which is one or two letters. The first letter is always capitalized.

The Fifth Element (1997)
Tennessine. [Rn]7s 2 5f 14 6d 10 7p 5 [note] Praseodymium. [Xe]6s 2 4f 3. Oganesson. [Rn]7s 2 5f 14 6d 10 7p 6 [note] Notes on the Electron Configuration of particular elements: Dubnium: Value is a guess based on periodic table trend. Seaborgium: Value is a guess based on periodic table trend.

How To Use a Periodic Table
Figure 2.25 (a) Dimitri Mendeleev is widely credited with creating (b) the first periodic table of the elements. (credit a: modification of work by Serge Lachinov; credit b: modification of work by "Den fjättrade ankan"/Wikimedia Commons) By the twentieth century, it became apparent that the periodic relationship involved atomic numbers.

The Fifth Element (1997) Plot Summary IMDb
Chemistry 10 2.5 The Periodic Table Learning Objectives By the end of this section, you will be able to: State the periodic law and explain the organization of elements in the periodic table Predict the general properties of elements based on their location within the periodic table
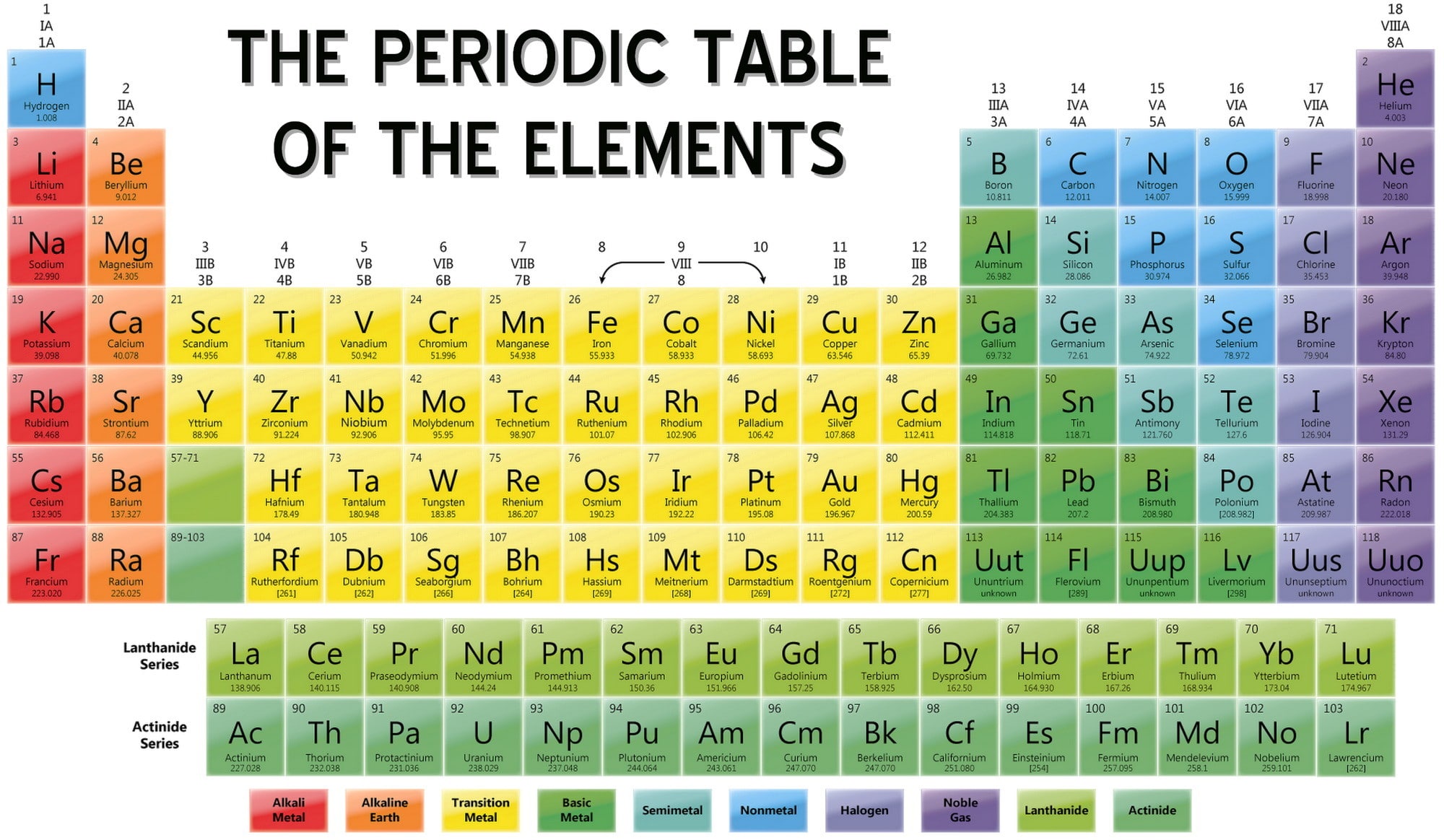
List Of Elements In Periodic Table Name And Symbol
2 He Helium 4.0026; 2. 3 Li Lithium 6.94; 4 Be Beryllium 9.0122; 5 B Boron 10.81; 6 C Carbon 12.011; 7 N Nitrogen 14.007; 8 O Oxygen 15.999; 9 F Fluorine 18.998; 10 Ne Neon 20.180; 3. 11 Na Sodium 22.990;. For elements with no stable isotopes, the mass number of the isotope with the longest half-life is in parentheses. View Wide Dark About.
/complete-periodic-table-of-elements-royalty-free-vector-166052665-5a565f0e47c2660037ab8aca.jpg)
List of Halogens (Element Groups)
Periodic Table of Elements TABLE LIST W/PROPERTIES GAME Display Property/Trend 17 Cl Chlorine halogen Plot Atomic Mass 1 H Hydrogen nonmetal 2 He Helium noble gas 3 Li Lithium alkali metal 4 Be Beryllium alkaline earth metal 5 B Boron metalloid 6 C Carbon nonmetal 7 N Nitrogen nonmetal 8

Elements Compounds And Mixtures Mini Chemistry Learn Chemistry Online Riset
This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. Typically, you need at least 8 steps to determine the electron configuration, starting with finding the atomic number by looking at the list of orbitals and understanding the notation.. But wait — you can avoid all this if you use our tool!
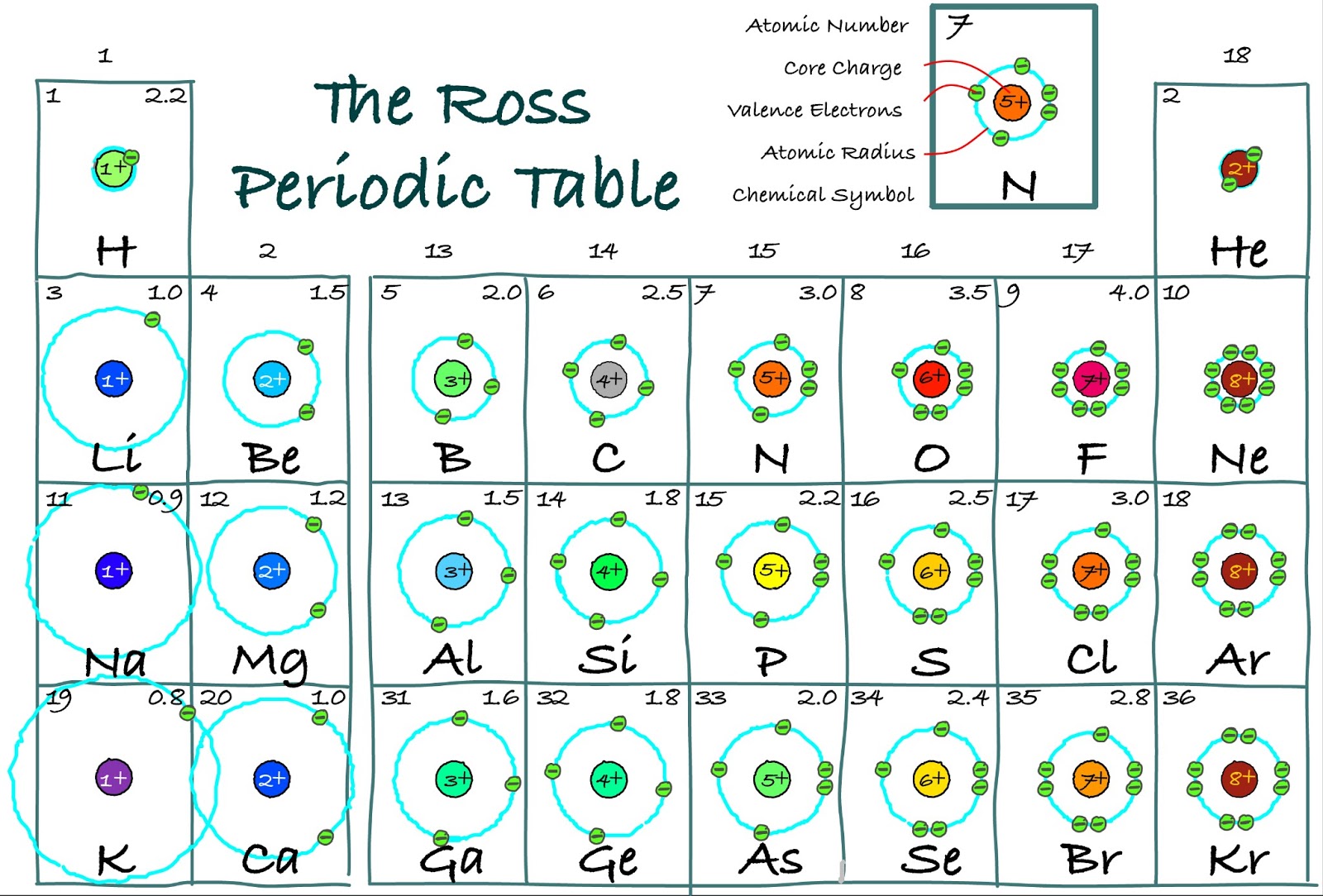
The Ross Periodic Table Core Charge Its Periodicity Across the Table
Updated on February 01, 2021 The electron configuration of an atom of any element is the of electrons per sublevel of the energy levels of an atom in its ground state . This handy chart compiles the electron configurations of the elements up through number 104. Key Takeaways: Electron Configurations

The Fifth Element Museum of Arts and Design
The next element is a boron with 5 electrons and the electron configuration 1s 2 2s 2 2p 1. Carbon has 6 electrons with the electron configuration 1s 2 2s 2 2p 2 . Remember that the s subshell has one orbital and can take a maximum of two electrons, but the p subshell has three orbitals and can take a maximum of six electrons, i.e., two per.