I3 Lewis Structure How To Discuss
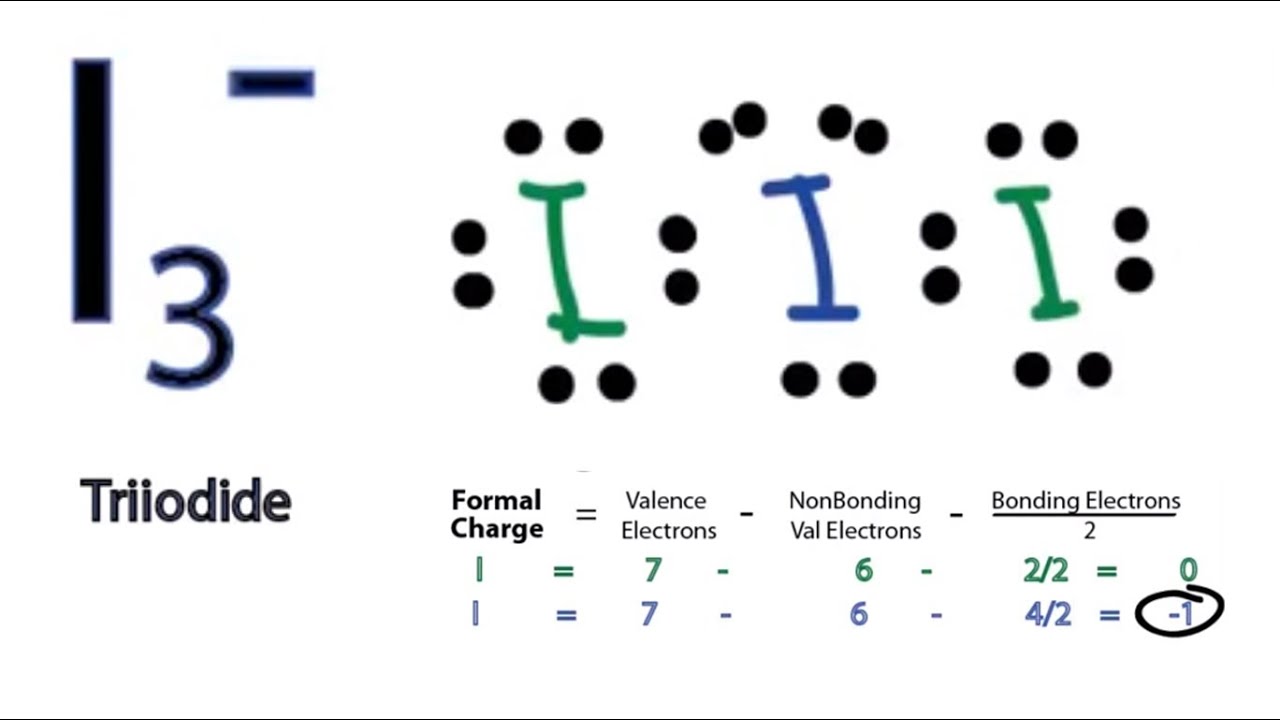
Lewis structure of I3- ion (triiodide) contains two single bonds between each Iodine (I) atom. All the three Iodine atoms have three lone pairs on it, and the central iodine atom has -1 formal charge. Let's draw and understand this lewis dot structure step by step.

SOLVED . Draw the Lewis structure of the following organic compounds
A step-by-step explanation of how to draw the I3 - Lewis Dot Structure (Triiodide Ion).For the I3 - structure use the periodic table to find the total number.

I3 Color Chemistry All Red Mania
The triiodide ion (I3-) is an anion that is formed by combining three iodine atoms. It is a common reagent in chemistry, often used in redox reactions and as an indicator for starch in iodometry. The ion has a linear shape, with the three iodine atoms arranged in a straight line. Drawing the Lewis Structure of I3-
Murph's Blog of Chem Joy SCH 3U VSEPR Theory
It is helpful if you: Try to draw the I 3- Lewis structure before watching the video. Watch the video and see if you missed any steps or information. Try structures similar to I 3- for more practice. List of Lewis Structures Lewis Structures for I3-. Step-by-step tutorial for drawing the Lewis Structure for I3-.

Lewis Dot Structure of I3 (Triiodide Ion) YouTube
An explanation of the molecular geometry for the I3 - ion (Triiodide Ion) including a description of the I3 - bond angles. The electron geometry for the Trii.
Get I3 Lewis Structure Molecular Geometry most complete GM
Contents show Lewis Structure of I3 Anyone wanting to know in-depth about a molecule needs to learn about the Lewis Structure. Why is it so necessary to have an idea on this topic? Well, Lewis Structure is the name given to the diagrammatic representation of chemical bonding occurring between atoms inside a molecule.
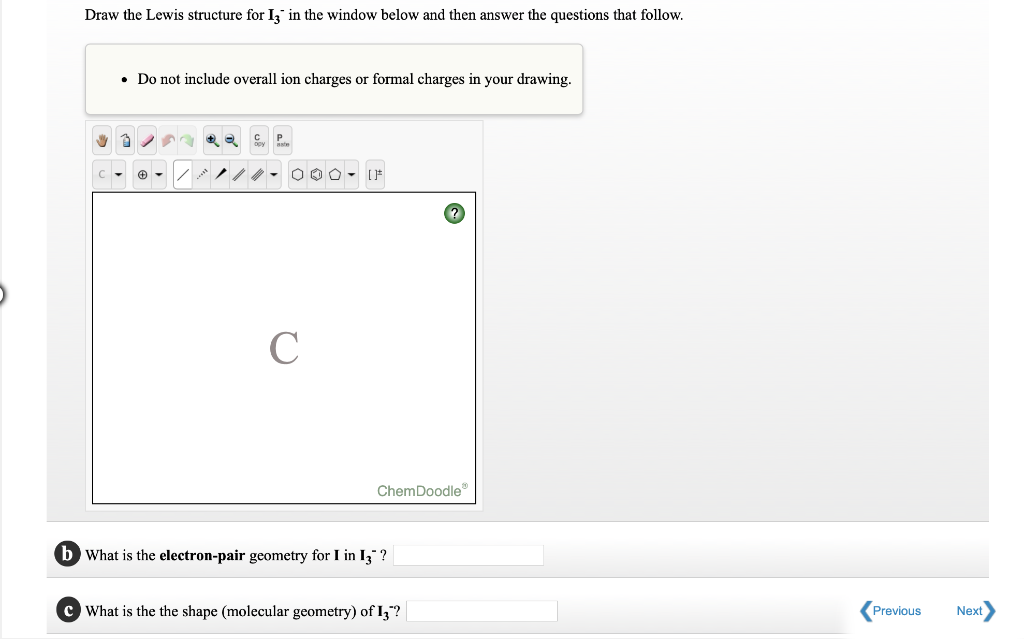
Solved Draw the Lewis structure for I3 in the window below
I quickly take you through how to draw the Lewis Structure of I3- (TriIodide Ion). I also go over hybridization, shape and bond angle.

Number of Lone Pairs and Bonding Pairs for I3 YouTube
Lewis Structures. Page ID. A Lewis Structure is a very simplified representation of the valence shell electrons in a molecule. It is used to show how the electrons are arranged around individual atoms in a molecule. Electrons are shown as "dots" or for bonding electrons as a line between the two atoms. The goal is to obtain the "best" electron.
Draw the Lewis dot structure of I3 Take a picture of … SolvedLib
Triiodide in Chemistry usually refers to the Triiodide ion, I3- This anion, one of the polyhalogen ions, is composed of 3 iodine atoms and is formed by combining the aqueous solution of iodine and iodide salts. A few salts of the anion have been isolated, including ammonium Triiodide ( [NH4]+[I3]− and thallium (I) Triiodide (Tl+ [I3]−)).
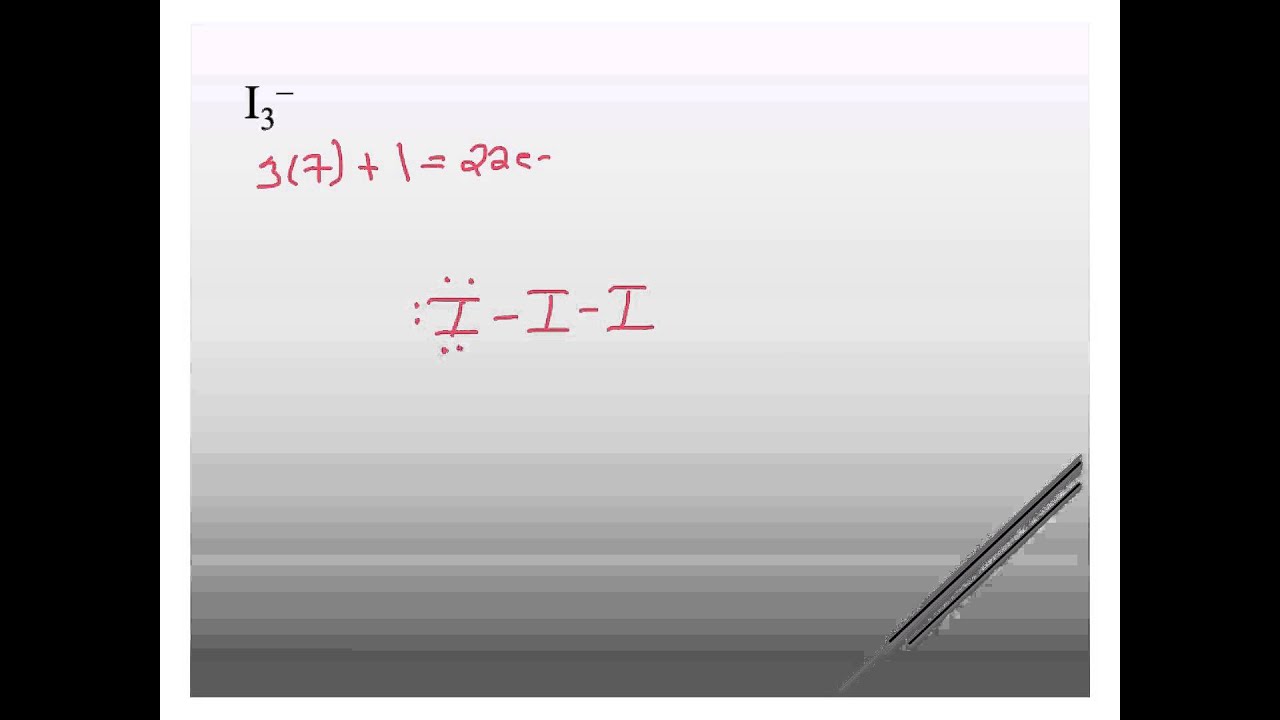
I3 Lewis structure YouTube
3. -. ) - Lewis Structure. In the lewis structure of triiodide ion (I 3- ), there are two I-I bonds. and one iodine atom is located as the center atom. Each iodine atom has 3 lone pairs and center iodine atom have -1 charge. We will learn how to draw the lewis structure of I 3- step by step in this tutorial.

Lewis Structure of I3 (triiodide ion) YouTube
I3- Lewis structure or lewis dot structure is a simple representation of the electronic structure of a molecule that briefs about the number of bonds formed, number of bond pairs required to fulfill the octet rule and lone pairs available.

to Chem Number of lone pair present at
Hello Guys,We are back with one of the most requested videos on Geometry of Molecules- I3- Lewis structure. It is a chemical formula for the Triiodide ion. T.

I3 Lewis Structure Triiodide Ion YouTube
I3- Lewis Structure, Shape, Hybridization and Polarity Written by Priyanka in Lewis Structure It is important to know the Lewis structure of a molecule to understand its physical properties, hybridization, and shape of the molecule.
I3 Lewis Structure How to Draw the Lewis Structure for I3 YouTube
Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.
[Solved] 1.Which of the options below ranks the following bonds from
Drawing the Lewis Structure for I 3-Viewing Notes: I 3-has a negative charge (and is therefore a negative ion or anion). That means that it has an extra electron that needs to be taken into account.. Let's do the I3- Lewis structure. On the periodic table, Iodine is in group 7 or 17. It has seven valence electrons. But we have three of them.

Gallery For > I3 Lewis Structure Molecular geometry, Vsepr theory
This chemistry video tutorial explains how to draw the lewis structure of I3-. It also discusses the molecular geometry, bond angle, hybridization, and formal charges of the triiodide ion..