
Water! The benefits of drinking enough (and how to do it!)
The triple point of water, T 3 = 273.16 K, is the standard fixed-point temperature for the calibration of thermometers. This agreement also sets the size of the kelvin as 1/273.16 of the difference between the triple-point temperature of the water and absolute zero. The phase diagram of water is a pressure-temperature diagram for water that.

The Water Droplets Free Stock Photo Public Domain Pictures
Tuesday, January 23 6:30 p.m. to 7:30 p.m. Every day, Clackamas Water Environment Services (WES) cleans more than 10 million gallons of wastewater at its Tri-City Water Resource Recovery Facility.

Phase diagram with a triple point O of water analogy. Download
In thermodynamics, the triple point of a substance is the unique combination of temperature and pressure at which solid phase, liquid phase, and gaseous phase can all coexist in thermodynamic equilibrium. By international agreement, the triple point of water has been assigned a value of 273.16 K (0.01 °C; 32.02 °F) and a partial vapor.

Huge Water Slide Coming to the TriCities July 23rd, 2106
Hundreds of thousands of West Virginians were left without clean water after a chemical leak got into the Elk River on Jan. 9, 2014. Elaine Tanner told Eyewitness News she tried to get bottles of.

HUNTS POINT WATERFRONT Mysite
The triple point occurs where the solid, liquid, and gas transition curves meet. The triple point is the only condition in which all three phases can coexist.
Water Drops Free Stock Photo Public Domain Pictures
Pure water has a triple point of 0. 01 ° C (273. 16 K, 32. 01 ° F) and pressure of 4. 58 mm (611. 2 Pa). Boiling point. The boiling point is defined as the point at which liquid starts boiling. At this point, matter exists in two states known as gaseous and liquid. The boiling point of water is 100 ° C. Difference between Boiling point and.

B603CL Baritone Saxophone LIEN CHENG SAXOPHONE CO., LTD.
The triple point occurs where the solid, liquid, and gas transition curves meet. The triple point is the only condition in which all three phases can coexist, and is unique for every material. Water reaches its triple point at just above freezing (0.01° C) and at a pressure of 0.006 atm. Related reading: Thermodynamic equilibrium.
Our Properties Water Front Properties
At a triple point (or triple line) P = 3 P = 3. For a two-component system like a mixture of ethanol and water, C = 2 C = 2. If we apply the equation, then we find that in this case, F = 1 F = 1, i.e. there is one degree of freedom. If we pick P P then T T is defined for us by the constraints of any two-component, three-phase system.
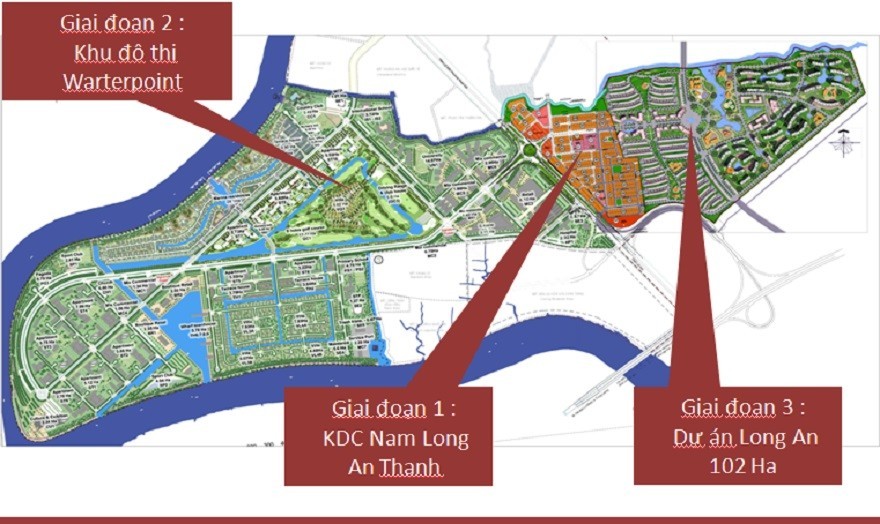
WaterPoint Bến Lức tận hưởng tiện ích đẳng cấp/htland.vn/
ABSTRACT. This paper is a part of guidelines, prepared on behalf of the Consultative Committee for Thermometry, on the methods how to realize the International Temperature Scale of 1990. It discusses all major issues linked to the triple point of water when used as a fixed point for the realization of the kelvin. 1. Introduction.

Water Point Hip Yick Industrial Company Limited
The triple point occurs where the solid, liquid, and gas transition curves meet. The triple point is the only condition in which all three phases can coexist, and is unique for every material. Water reaches its triple point at just above freezing (0.1° C) and at a pressure of 0.006 atm. Notes: Currently only transportable to Thimann Lecture halls.

Combining Proven Brands to Provide Our Customers with Reliable
Triple point of water. In chemistry and physics, the triple point is the temperature and pressure at which solid, liquid, and vapor phases of a particular substance coexist in equilibrium. It is a specific case of thermodynamic phase equilibrium. The term "triple point" was coined by James Thomson in 1873.

Water Plunge Free Stock Photo Public Domain Pictures
Triple point of water is 273 K temperature and 0.46 cm of mercury pressure. The triple point of water, T 3 = 273.16 K, is the standard fixed-point temperature for the calibration of thermometers. This agreement also sets the size of the kelvin as 1/273.16 of the difference between the triple-point temperature of water and absolute zero.

TriValley Water Agencies Mandatory Water Usage Restrictions Remain in
TRIPLE-POINT CELL is a vacuum-tight flask containing water vapor, liquid water and an ice mantle that has formed around an inner test tube. To reach the triple point, first chill the cell.

Freehold Rum Point Water Fron
The Mathematics of Triple Point. The triple point of water is defined to take place at 273.16 K, where K is the SI unit Kelvin. Unlike Celsius and Fahrenheit scales, Kelvin is not measured using degrees; we merely say "Kelvin.". The triple point of water is important enough to merit its own line: T3 = 273.1K.

Vitapur Pointof Use TriTemperature Water Dispenser The Home Depot
The triple point of water is at 273.16 kelvin (0.01 °C or 32.02 °F) and a pressure of 611.7 pascals (6.117 millibars, 0.0060373057 atm). (Some sources list a pressure of 611.73 Pa, while others cite a pressure of 611.657 Pa.) At this point, ice, water, and water vapor coexist in stable form. The tiniest change in temperature or pressure.

FileRaindrops on water.jpg Wikimedia Commons
There are over 40 million people under a severe storm threat on Tuesday, according to the latest from the Storm Prediction Center. An enhanced risk for severe storms, or a level 3 of 5, is in.