
27 Bohr Diagram For Calcium Wiring Database 2020
Steps Here's how you can draw the Bohr model of calcium step by step. #1 Write protons, neutrons, and electrons of calcium atom #2 Draw nucleus of calcium atom #3 Draw 1 st electron shell #4 Draw 2 nd electron shell #5 Draw 3 rd electron shell #6 Draw 4 th electron shell Let's break down each step in detail.
[DIAGRAM] Bohr Model Diagram For Calcium
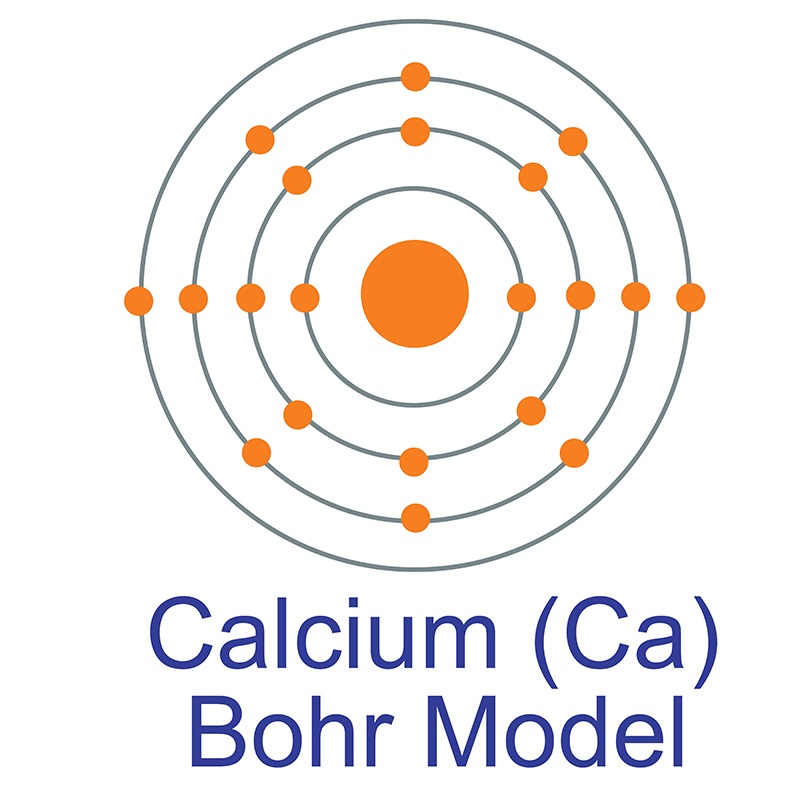
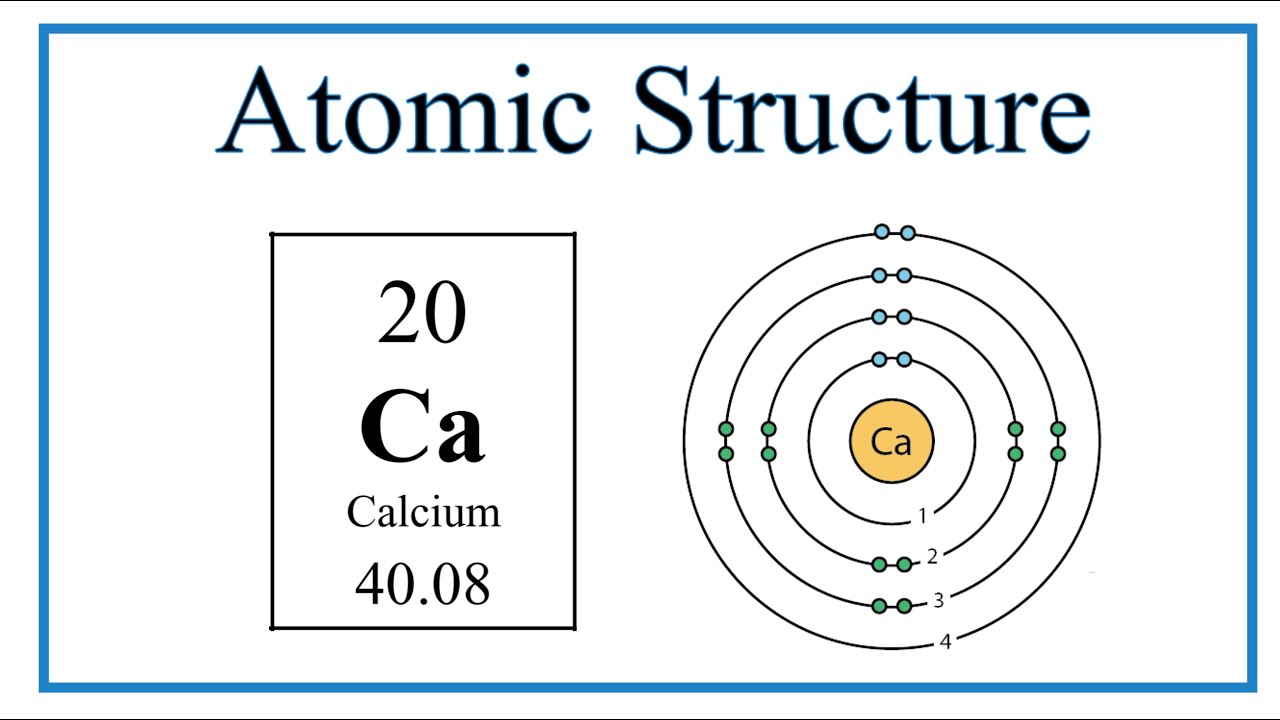
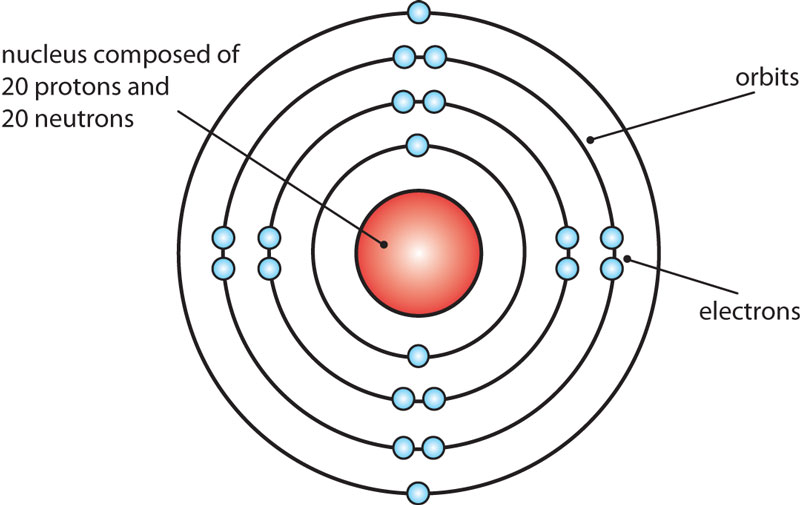
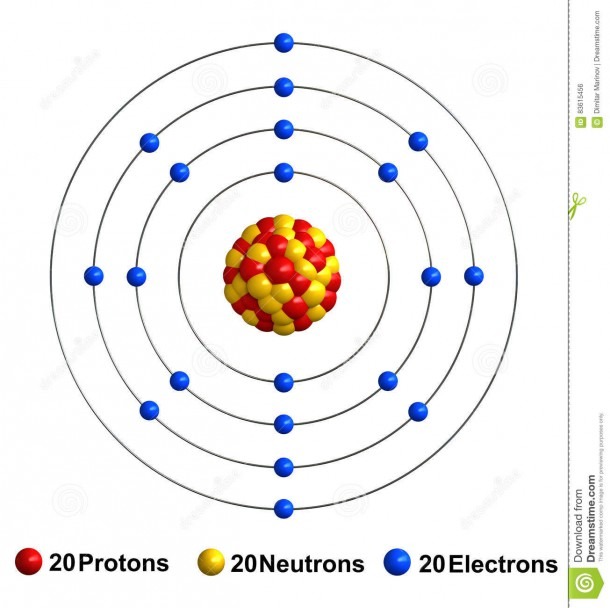
Name: Calcium Symbol: Ca Atomic Number: 20 Atomic Mass: 40.078 amu Melting Point: 839.0 °C (1112.15 K, 1542.2 °F) Boiling Point: 1484.0 °C (1757.15 K, 2703.2 °F) Number of Protons/Electrons: 20 Number of Neutrons: 20 Classification: Alkaline Earth Crystal Structure: Cubic Density @ 293 K: 1.55 g/cm 3 Color: Silvery Atomic Structure

27 Bohr Diagram For Calcium Wiring Database 2020
Calcium has 2 electrons in its first shell, 8 in its second, 8 in its third, and 2 in its fourth.Check me out: http://www.chemistnate.com

Bohr Diagram Of Calcium
One of the weaknesses of Bohr's model was that he could not offer a reason why only certain energy levels or orbits were allowed. Figure 10.4.1 10.4. 1: The energy levels of the electrons can be viewed as rungs on a ladder. Note that the spacing between rungs gets smaller at higher energies (CC BY-NC; Ümit Kaya)
Calcium Phosphate Nanoparticles AMERICAN ELEMENTS
Bohr model of calcium: (CC BY-SA 2.0 uk;Greg Robson): Answer b. Bohr model of sulfur: (CC BY-SA 2.0 uk; Greg Robson). Valence electrons are located in the highest energy level of an atom. When drawing a Bohr diagram, the valence electrons would be present in the outermost electronic level/shell (furthest away from the nucleus). An atom can have.
Bohr Diagram For Calcium Wiring Diagram
Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Figure 2 2 contrast the Bohr diagrams for lithium, fluorine and aluminum atoms. The shell closest to the nucleus is.
Calcium Shell Model
A Visual Representation: A Bohr model is a way of visually representing what an atom of an element looks like. Atoms are tiny particles that make up matter and they have within them protons, neutrons and electrons. Neils Bohr, a Danish scientist, found that electrons orbit around the nucleus of the atom in various energy levels.
اتم چیست؟ سوالی در مورد فیزیک! مدیاسافت
Why is the valency of the Calcium (Ca) Bohr model, set to 2 electrons, instead of 1 electron for stability?. $\begingroup$ @Karl instructor went straight to drawing the Bohr model,. Aug 30, 2019 at 1:54 $\begingroup$ Well, Bohr is only step one. It seems you have progressed now, keep it up! $\endgroup$ - Karl. Aug 30, 2019 at 6:33. Add.
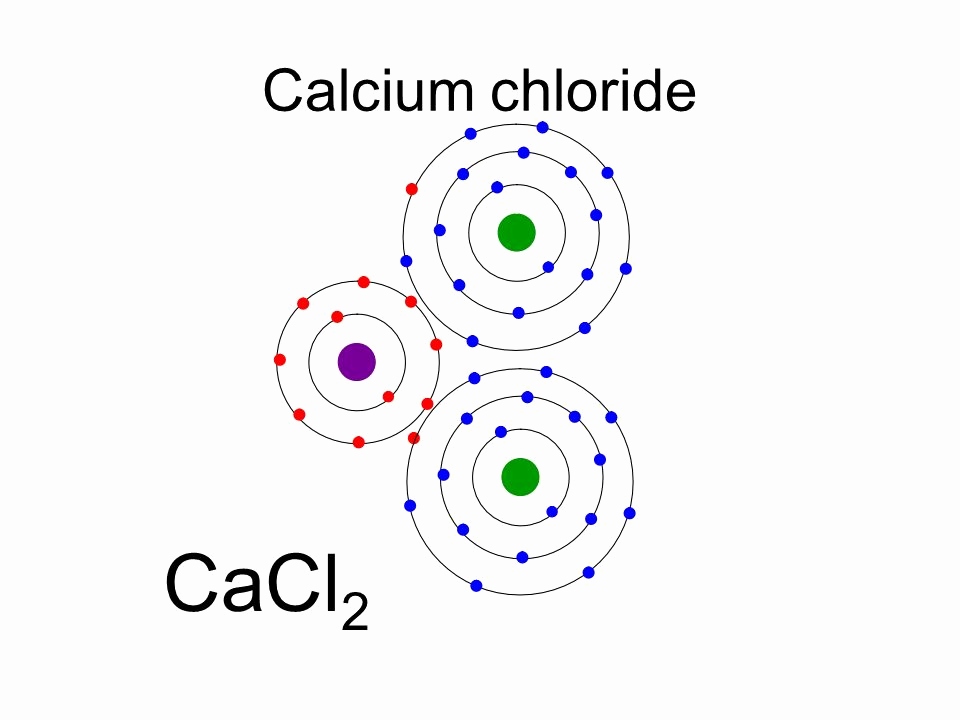
Calcium Bohr diagram Calcium
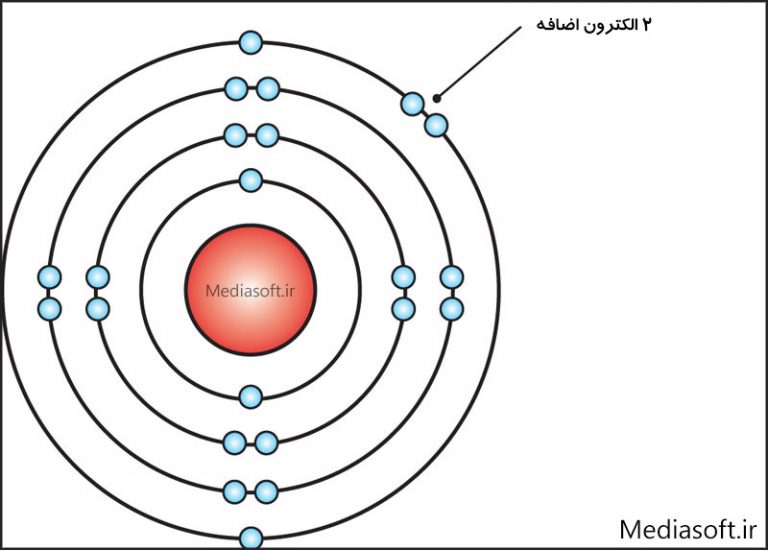
The elements that form bonds by donating electrons are called cation. Calcium donates two electrons of the last shell to form bonds and turns into a calcium ion (Ca 2+ ). That is, calcium is a cation element. Ca - 2e - → Ca 2+. The electron configuration of calcium ion (Ca 2+) is 1s 2 2s 2 2p 6 3s 2 3p 6.
Bohr Diagram Of Calcium
Learn how to draw Bohr Models of atoms to further your fundamental understanding of Chemistry.

Calcium Bohr Diagram
The Bohr Model has an atom with a positively-charged nucleus surrounded by negatively-charged electrons that have circular, planetary-like orbits. Today, we know that the Bohr Model has some inaccuracies, but it's still used because of its simple approach to atomic theory.
Bohr Model Calcium
The Bohr model of calcium is a simplified representation of the atom's nuclear structure, named after Danish physicist Niels Bohr. It depicts the nucleus as a small, positively charged ball with electrons orbiting around it in circular paths at fixed distances.
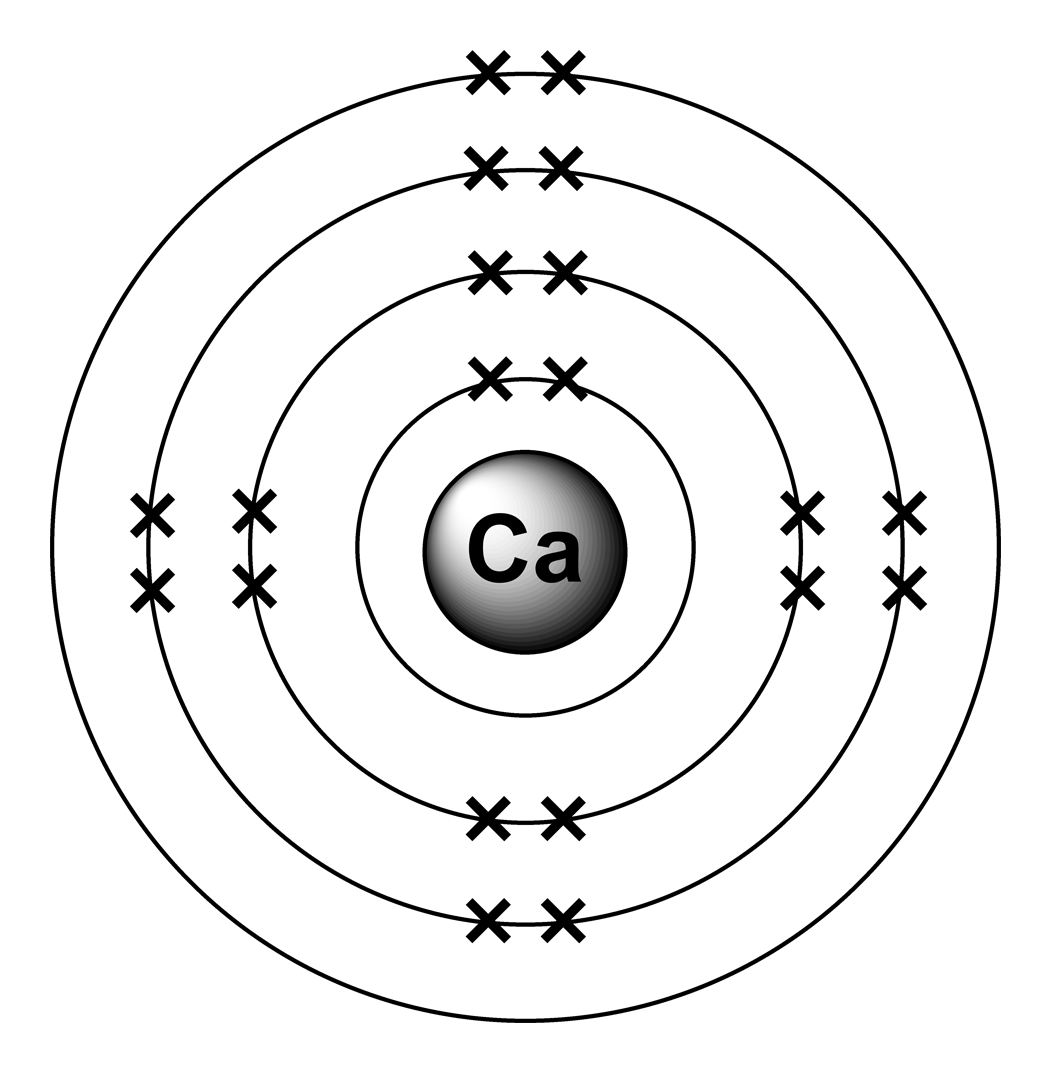
Bohr Diagram Of Calcium
The Bohr Model of Calcium (Ca) has a nucleus that contains 20 neutrons and 20 protons. This nucleus is surrounded by four-electron shells named K-shell, L-shell, M-shell, and N-shell. The outermost shell in the Bohr diagram of Calcium contains only 2 electrons that also called valence electrons. Page Contents show

How to Build a Model of a Calcium Atom Articles MerchantCircle
The electron volt (eV) is a convenient unit of energy for expressing atomic-scale energies. It is the amount of energy that an electron gains when subjected to a potential of 1 volt; 1 eV = 1.602 ×10−19 J 1 e V = 1.602 × 10 − 19 J. Using the Bohr model, determine the energy, in electron volts, of the photon produced when an electron in a.
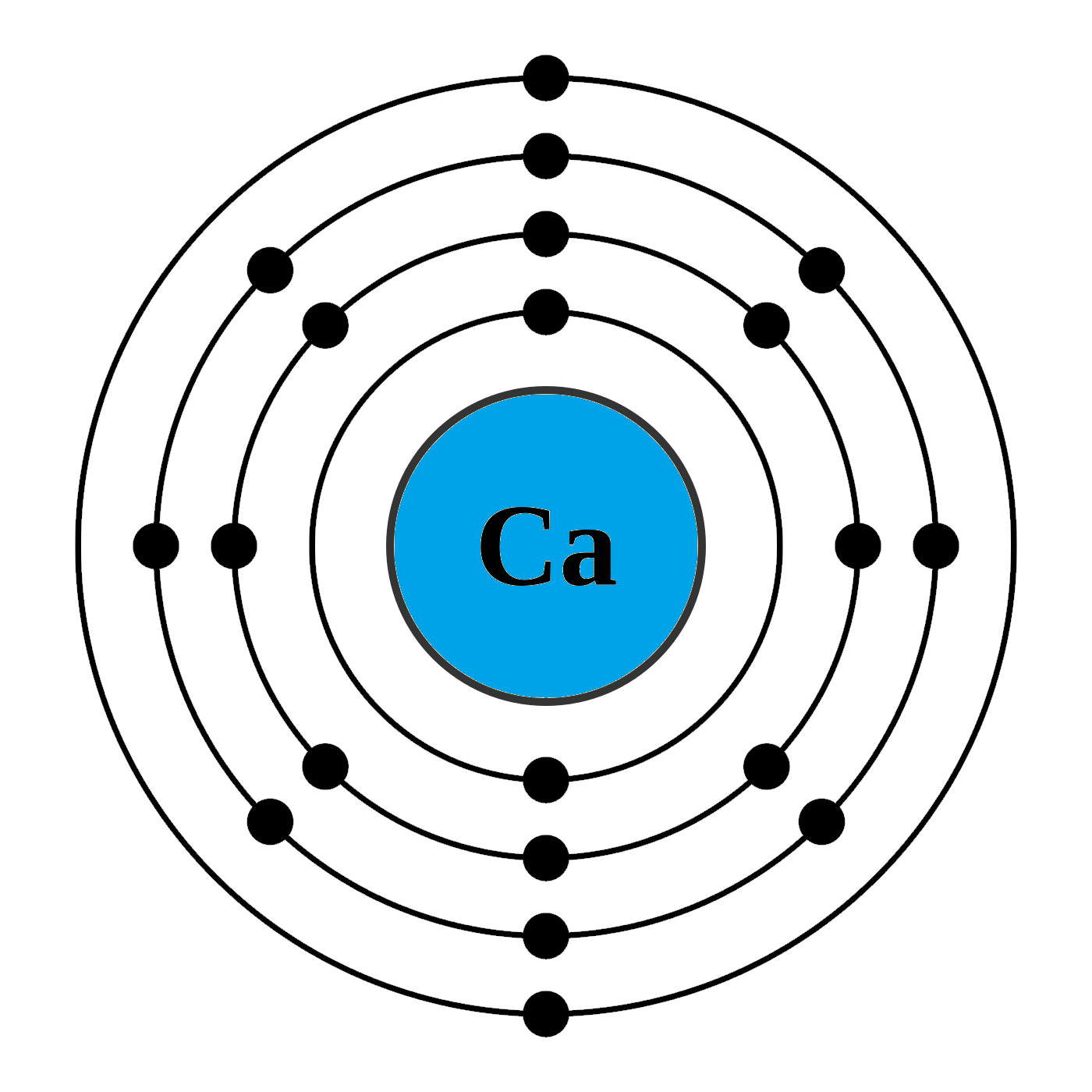
How can I draw electronic configuration of calcium in a shell nxwe70dd
Immediately before 1913, the Rutherford model conceived of an atom as consisting of a tiny positively charged heavy core, called a nucleus, surrounded by light, planetary negative electrons revolving in circular orbits of arbitrary radii. Britannica Quiz Matter and More Quiz How does Niels Bohr's atomic model work?

Bohr Model Manganese Atom Electron Structure เวกเตอร์สต็อก (ปลอดค่า
Course: Class 9 Chemistry (India) > Unit 4. Lesson 1: Models of an atom. Discovery of the electron and nucleus. Rutherford's gold foil experiment. Drawback of the Rutherford model. Bohr's model of an atom. Atomic structure. Science >. Class 9 Chemistry (India) >.